
Chemistry
Higher and Standard level
Specimen papers 1A, 1B and 2
For first examinations in 2025
CONTENTS
Chemistry higher level paper 1A specimen question paper
Chemistry higher level paper 1A specimen markscheme
Chemistry higher level paper 1B specimen question paper
Chemistry higher level paper 1B specimen markscheme
Chemistry higher level paper 2 specimen question paper
Chemistry higher level paper 2 specimen markscheme
Chemistry standard level paper 1A specimen question paper
Chemistry standard level paper 1A specimen markscheme
Chemistry standard level paper 1B specimen question paper
Chemistry standard level paper 1B specimen markscheme
Chemistry standard level paper 2 specimen question paper
Chemistry standard level paper 2 specimen markscheme

© International Baccalaureate Organization 2023
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
14 pages
Specimen paper
2 hours [Paper 1A and Paper 1B]
Chemistry
Higher level
Paper 1A
Instructions to candidates
y Do not open this examination paper until instructed to do so.
y Answer all questions.
y For each question, choose the answer you consider to be the best and indicate your choice on
the answer sheet provided.
y A calculator is required for this paper.
y A clean copy of the chemistry data booklet is required for this paper.
y The maximum mark for paper 1A is [40 marks].
y The maximum mark for paper 1A and paper 1B is [75 marks].
– 2 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
Section A
1. What is thin-layer chromatography best used for separating?
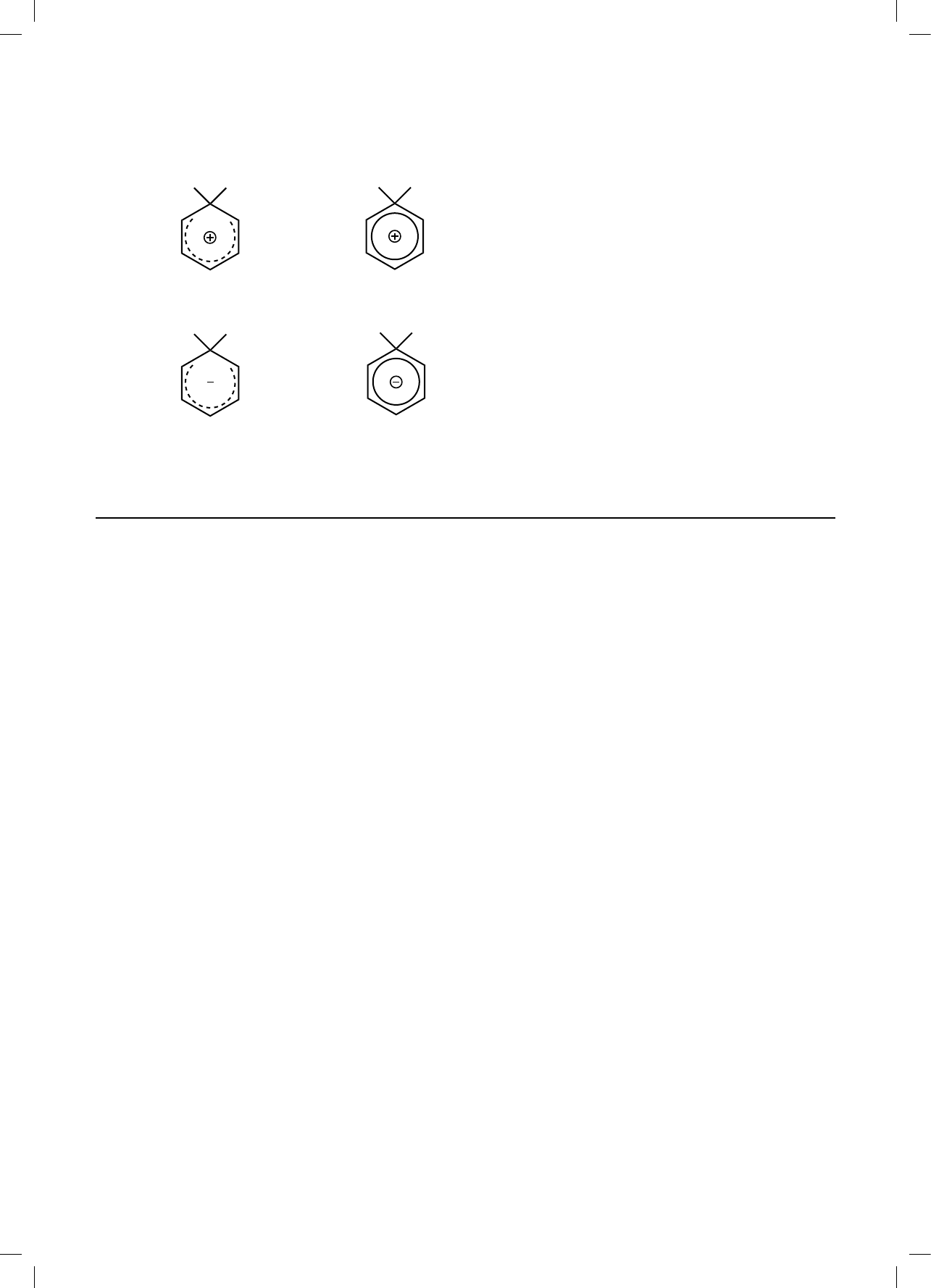
A. molecules of varying polarity
B. molecules of similar polarity
C. metals in an alloy
D. water of crystallization from hydrated salts
2. Ice containing only the isotope
2
H sinks and does not melt when dropped into ordinary
distilled water maintained at 3
C.
Which statement is correct?
A. The isotope
2
H has a high natural abundance.
B.
2
H
2
O (s) has a higher melting point than normal ice.
C.
2
H
2
O (s) has a lower density than normal ice-cold water.
D.
2
H
2
O has different chemical properties from normal water.
3. The table lists successive ionization energies of an element Z.
Ionization number 1st 2nd 3rd 4th 5th 6th
Ionization energy / kJ mol
−
−
1
577.54 1816.68 2744.78 11 577.5 14 841.9 18 379.0
Which is the formula of the stable oxide of the element Z?
A. Z
2
O
B. ZO
C. Z
2
O
3
D. ZO
2

– 3 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
Turn over
4. A container holds 30 g of argon and 60 g of neon.
What is the ratio of number of atoms of argon to number of atoms of neon in the container?
A. 0.25
B. 0.50
C. 2.0
D. 4.0
5. A gas storage tank of fixed volume V contains N molecules of an ideal gas at 300 K with a
pressure of 40 kPa.
N
4
molecules are removed, and the temperature is changed to 450 K.
What is the new pressure of the gas in kPa?
A. 15
B. 30
C. 45
D. 60
6. What is the formula of the compound formed between magnesium ions and
hydrogencarbonate ions?
A. MgHCO
3
B. Mg(HCO
3
)
2
C. Mg(HCO
3
)
3
D. Mg
3
(HCO
3
)
2
7. In which group of ions and molecules are electrons delocalized in all species?
A. CH
3
COOH, O
3
, C
60
B. CH
3
COO
−
, NO
2
−
, C(graphite)
C. C
2
H
2
, (COOH)
2
, C(diamond)
D. C
2
H
4
, NO
2
+
, SiO
2

– 4 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
8. What is the molecular geometry of the central atom in SF
4
Cl
2
?
A. linear
B. tetrahedral
C. hexagonal
D. octahedral
9. Which is the preferred Lewis formula of nitrous oxide, N
2
O, as deduced by formal charges?
A.
N N
O
B.
N N
O
C.
N N
O
D.
N
O
N
10. Which pair of statements about electrons in this molecule is correct?
O
H
O
N
H
H
Number of non-bonding
pairs of electrons
Number of electrons in
π
π bonds
A.
3 6
B.
3 8
C.
5 6
D. 5 8
11. What is the explanation for the malleability of metals?
A. The bonds are strong.
B. The bonds are weak.
C. The bonds involve free electrons.
D. The bonds do not have a specific direction.

– 5 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
Turn over
12. The structure shows the repeating unit of a polymer found in some plastics.
CH
2
C
CH
3
CH
3
Which monomer is used to form this plastic?
A. H
2
C=C(CH
3
)
2
B. CH
3
CH(CH
3
)
2
C. (H
3
C)
2
C=C(CH
3
)
2
D. (H
3
C)
2
C=CHCH(CH
3
)
2
13. Why is copper(II) sulfate blue?
A. Red light is absorbed when electrons are promoted between the orbitals in the split
d-sublevels.
B. Blue light is emitted when electrons fall between the orbitals in the split d-sublevels.
C. Red light is absorbed when electrons fall between the orbitals in the split d-sublevels.
D. Blue light is emitted when electrons are promoted between the orbitals in the split
d-sublevels.
14. Which molecule has a carbonyl functional group?
A. CH
3
OCH
3
B. CH
3
COCH
3
C. CH
3
CH
2
OH
D. CH
3
CH
2
NH
2
15. The block structure of the periodic table groups elements according to which characteristic?
A. atomic number
B. atomic mass
C. electron configuration
D. reactivity

– 6 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
16. Which d block element has the highest number of different oxidation states?
A. Ti
B. Mn
C. Cu
D. Zn
17. What do all greenhouse gases have in common?
A. They are emitted by the burning of fossil fuels.
B. They absorb ultraviolet radiation.
C. They are symmetrical molecules with no polar bonds.
D. They absorb infrared radiation.
18. Which is the
1
H NMR spectrum of cyclohexane?
A.
8 6 4 2
0
Chemical shift / ppm
B.
8 6 4 2
0
Chemical shift / ppm
C.
8 6 4 2
0
Chemical shift / ppm
D.
8 6 4 2
0
Chemical shift / ppm

– 7 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
Turn over
19. Which is the mass spectrum of butanal?
A.
0 15 30 45 60 75
m/z
100
80
60
40
20
0
Relative intensity
B.
0 15 30 45 60 75
m/z
100
80
60
40
20
0
Relative intensity
C.
0 15 30 45 60 75
m/z
100
80
60
40
20
0
Relative intensity
D.
0 15 30 45 60 75
m/z
100
80
60
40
20
0
Relative intensity
20. The potential energy profile for a “coffee cup” calorimetry experiment is shown.
Thermometer
Coffee cup calorimeter
Reactants
Products
Reaction coordinate
Potential energy
What is the correct interpretation of this reaction?
Temperature Type of reaction
A. increases exothermic
B. increases endothermic
C. decreases exothermic
D. decreases endothermic

– 8 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
21. What is the enthalpy change for the reaction in kJ mol
−
1
?
CO
2
(g) + H
2
(g) → CO (g) + H
2
O (g)
2CO (g) + O
2
(g) → 2CO
2
(g) ΔH = −566 kJ mol
−
1
2H
2
(g) + O
2
(g) → 2H
2
O (l) ΔH = −572 kJ mol
−
1
H
2
O (g) → H
2
O (l) ΔH = −44 kJ mol
−
1
A. −1182
B. −899
C. −41
D. +41
22. Which is a renewable energy source?
A. natural gas
B. uranium
C. coal
D. wood
23. Which are endothermic processes in a Born–Haber cycle for the formation of an ionic
compound?
I. Enthalpy of atomization
II. First electron affinity
III. First ionization energy
A. I and II only
B. I and III only
C. II and III only
D. I, II and III

– 9 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
Turn over
24. What is correct as a system approaches equilibrium?
A. Q remains constant.
B. K
c
increases.
C. ΔG
Ö
becomes more negative.
D. ΔG approaches zero.
25. The complete combustion of 20.0 cm
3
of a gaseous hydrocarbon, C
x
H
y
, produces 80.0 cm
3
of gaseous products. This volume reduces to 40.0 cm
3
when the water vapour present
condenses. All volumes are measured at the same temperature and pressure.
What is the molecular formula of the hydrocarbon?
A. CH
4
B. C
2
H
2
C. C
2
H
4
D. C
3
H
6
26. The diagram shows the energy profile of a reaction.
Reactants
Products
Reaction coordinate
Potential energy
X
Y
Z
Which combination is correct?
Activation energy of
forward reaction
Activation energy of
reverse reaction
A. X Z
B.
Y − X Y − Z
C. Y Y
D.
Y − X Z − X

– 10 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
27. What is the main reason for an increase in rate of reaction when the temperature is raised?
A. A greater proportion of collisions are successful.
B. Particles collide more frequently.
C. The bonds in the reactants are weakened.
D. The activation energy of the reaction decreases.
28. What is the equilibrium constant expression for the following reaction?
2SO
3
(g) 2SO
2
(g) + O
2
(g)
A.
[SO][O ]
[SO]
2
2
2
3
2
B.
[SO] [O ]
[SO]
2
2
2
3
2
+
C.
[SO]
[SO][O ]
3
2
2
2
2
D.
2[SO ][O ]
2[SO ]
22
3
29. Which statement is correct about points X and Y on the energy profile diagram?
Reaction coordinate
Potential energy
Reactants
Products
X
Y
A. X is a transition state and Y is an intermediate.
B. X is an intermediate and Y is a transition state.
C. X and Y are transition states.
D. X and Y are intermediates.

– 11 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
Turn over
30. The equation for the reaction between two gases, A and B, is:
A (g) + 2B (g) 2C (g)
When the reaction is at equilibrium at 600 K, the concentrations of A, B, and C are 2, 1, and
2 mol dm
−
3
respectively. What is the value of the equilibrium constant at 600 K?
A. 0.25
B. 1
C. 2
D. 4
31. Which reaction would be expected to have the largest Arrhenius (pre-exponential) factor, A,
at constant temperature?
A. H (g) + I (g) → HI (g)
B. H
2
(g) + I
2
(g) → 2HI (g)
C. 2HCl (g) → H
2
(g) + Cl
2
(g)
D. H
2
+ C
2
H
4
→ C
2
H
6
32. Which reactions involve the transfer of a proton?
I. 2HCl (aq) + Mg (s) → MgCl
2
(aq) + H
2
(g)
II. 2HCl (aq) + MgO (s) → MgCl
2
(aq) + H
2
O (l)
III. 2HCl (aq) + MgCO
3
(s) → MgCl
2
(aq) + H
2
O (l) + CO
2
(g)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III

– 12 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
33. The indicator, HInd, is used in an acid–base titration.
HInd (aq) H
+
(aq) + Ind
−
(aq)
colour A colour B
Which statements are correct?
I. In a strongly alkaline solution, colour B is observed.
II. Colour A is observed when [HInd] < [Ind
−
].
III. [Ind
−
] approximately equals [HInd] at the end point.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
34. The overall reaction occurring at the electrodes of a rechargeable metal hydride battery can
be summarized as:
MH + NiO(OH) M + Ni(OH)
2
Which statement is correct?
A. The oxidation state of Ni does not change.
B. M is oxidized by loss of hydrogen.
C. The oxidation state of one H atom changes from −1 to +1.
D. The oxidation state of one O atom changes from −1 to −2.
35. In a redox titration, manganate(VII) ions are reduced to manganese(II) ions and iron(II) ions
are oxidized to iron(III) ions.
MnO
4
−
(aq) reduced to Mn
2
+
(aq)
Fe
2
+
(aq) oxidized to Fe
3
+
(aq)
What volume, in cm
3
, of 0.1 mol dm
−
3
MnO
4
−
(aq) is required to reach the equivalence point in
the titration of 20.00 cm
3
of 0.1 mol dm
−
3
Fe
2
+
(aq)?
A. 2.00
B. 4.00
C. 20.00
D. 100.00

– 13 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
Turn over
36. Which statements explain the following reactions occurring in the upper atmosphere?
Chlorofluorocarbon (CFC) compounds
break down to produce chlorine radicals
but usually not fluorine radicals.
A single chlorine radical breaks down
many ozone, O
3
, molecules.
A.
C–Cl bond is stronger than C–F bond
chain propagation steps produce more
radicals
B.
C–F bond is stronger than C–Cl bond
chain termination steps cause chlorine
radicals to reform chlorine molecules
C.
C–Cl bond is stronger than C–F bond
chain termination steps cause chlorine
radicals to reform chlorine molecules
D.
C–F bond is stronger than C–Cl bond
chain propagation steps produce more
radicals
37. Which term cannot characterize ammonia, NH
3
?
A. Lewis acid
B. Brønsted–Lowry acid
C. ligand
D. nucleophile
38. Which ion is a better leaving group in nucleophilic substitutions?
A. bromide ion
B. chloride ion
C. fluoride ion
D. iodide ion
39. Which statement is correct when 2-chloro-2-methylpentane reacts with water to form
2-methylpentan-2-ol?
A. Water acts as a nucleophile and attacks the chlorine atom.
B. The reaction occurs in a single step.
C. A carbocation intermediate is formed.
D. Homolytic bond fission occurs.
– 14 –
SPEC/4/CHEMI/HPM/ENG/TZ0/XX
0000 – 6101
40. Which illustrates the correct intermediate formed in the nitration of benzene by NO
2
+
?
A.
H
NO
2
B.
H
NO
2
C.
H
NO
2
D.
H
NO
2

2 pages
Markscheme
Specimen paper
Chemistry
Higher level
Paper 1 – Section A
– 2 –
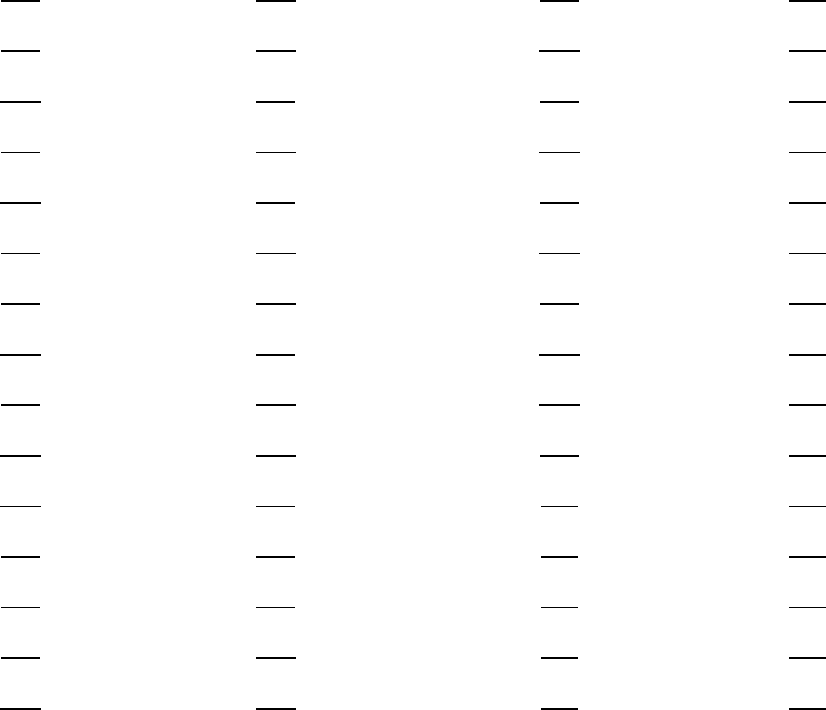
1. A 16. B 31. A 46. –
2. B 17. D 32. C 47. –
3. C 18. A 33. B 48. –
4. A 19. C 34. C 49. –
5. C 20. A 35. B 50. –
6. B 21. D 36. D 51. –
7. B 22. D 37. A 52. –
8. D 23. B 38. D 53. –
9. B 24. D 39. C 54. –
10. D 25. C 40. A 55. –
11. D 26. B 41. – 56. –
12. A 27. A 42. – 57. –
13. A 28. A 43. – 58. –
14. B 29. C 44. – 59. –
15. C 30. C 45. – 60. –

Candidate session number
© International Baccalaureate Organization 2023
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
11 pages
Specimen paper
2 hours [Paper 1A and Paper 1B]
Chemistry
Higher level
Paper 1B
Instructions to candidates
y Write your session number in the boxes above.
y Do not open this examination paper until instructed to do so.
y Answer all questions.
y Answers must be written within the answer boxes provided.
y A calculator is required for this paper.
y A clean copy of the chemistry data booklet is required for this paper.
y The maximum mark for paper 1B is [35 marks].
y The maximum mark for paper 1A and paper 1B is [75 marks].
12EP01

Please do not write on this page.
Answers written on this page
will not be marked.
– 2 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
12EP02

– 3 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
Turn over
Section B
Answer all questions. Answers must be written within the answer boxes provided.
1. Hypochlorous acid, HOCl, is a sterilizing agent used in swimming pools and is produced
when chlorine reacts with water.
Cl
2
(aq) + H
2
O (l) HOCl (aq) + HCl (aq)
(a) Deduce the oxidation states of chlorine in the reactants and products. [2]
Reactant: Cl
2
:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Products: HOCl:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
HCl:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Explain why more chlorine reacts with water when NaOH (aq) is added. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) Researchers studying the solubility of chlorine in pure water at different temperatures
compile the following data from different sources.
Source
Temperature /
C
Solubility of chlorine
A 0 1.46 g per 100 cm
3
B 10 310 cm
3
per 100 cm
3
C 20 0.70 g per 100 cm
3
D 25 6300 mg per 1000 cm
3
E 30 177 cm
3
per 100 cm
3
F 30 0.57 g per 100 cm
3
(This question continues on the following page)
12EP03

– 4 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
(Question 1 continued)
(i) Identify a problem in comparing the data from different sources. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) The units of solubility are converted to mol dm
−
3
.
Source
Temperature /
C
Solubility of chlorine Solubility of chlorine
/ mol dm
−
−
3
A 0 1.46 g per 100 cm
3
0.206
B 10 310 cm
3
per 100 cm
3
0.13
C 20 0.70 g per 100 cm
3
0.099
D 25 6300 mg per 1000 cm
3
0.089
E 30 177 cm
3
per 100 cm
3
F 30 0.57 g per 100 cm
3
0.080
Complete the table by calculating the value for source E.
Assume the density of chlorine is 2.86 g dm
−
3
at 30
C. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Suggest an explanation for the effect of temperature on solubility. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) Suggest why chlorine is not often added to swimming pools directly. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
12EP04

– 5 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
Turn over
(Question 1 continued)
(e) HOCl ionizes to form the hypochlorite ion, OCl
−
, which is a less effective disinfectant
than the undissociated acid.
The graph shows the concentrations of HOCl (aq) and OCl
−
(aq) at different pH values
at 25
C.
100
90
80
70
60
50
40
30
20
10
0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Fraction / %
pH
HOCl
OCl
−
(i) Deduce the pH range where the water is most effectively sterilized. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Determine, with reference to the graph in (e), the pK
a
of HOCl. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
12EP05

– 6 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
(Question 1 continued)
(f) Ammonia released from sweat and urine reacts with HOCl to form a range of
compounds including chloramines.
(i) Deduce an equation for the formation of dichloramine, NHCl
2
(aq), from ammonia
and HOCl (aq). [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) The graph shows the molar ratio of chloramines formed at different pH values at
25
C. Trichloramine, NCl
3
, causes pool water to smell bad.
NCl
3
NHCl
2
NH
2
Cl
100
75
50
25
0
2
3
4 5
6
7
8
Molar ratio / %
pH
State two conditions needed to prevent the bad smell. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(g) Suggest two reasons why operating a swimming pool at a lower temperature is
favourable for the environment. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12EP06

– 7 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
Turn over
2. A student investigates the effect of exposure to the air on the ascorbic acid (vitamin C)
concentration in a specific orange juice. Equal volumes of orange juice are sealed into
identical flasks and placed in a refrigerator for two weeks. The samples in the refrigerator are
exposed to the air by removing the stopper for a different number of hours each day as shown.
0 h 1 h 2 h 8 h 24 h
Stopper
Flask
Juice
(a) Identify two variables that are controlled. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) The concentration of ascorbic acid is determined by titration with a standard iodine
solution. Every few days, 10.00 cm
3
of orange juice is removed from each sample,
diluted to 100.0 cm
3
, and titrated.
(i) Suggest why the juice is diluted before titration. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Identify a possible systematic error with this method regarding the sample that is
exposed for zero hours. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Suggest how an additional flask could be set up to verify whether the systematic
error in (ii) has occurred. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
12EP07

– 8 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
(Question 2 continued)
(c) The following data are collected during a titration.
Final burette reading = 16.10 ± 0.05 cm
3
Initial burette reading = 1.10 ± 0.05 cm
3
Calculate the percentage uncertainty of the titre. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) The following graph shows the student’s results.
+
+
+
+
+
+
+
+
+
+
+
+
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
0.0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
4.5
5.0
5.5
6.0
0 h
1 h
2h
8 h
24 h
Time / d
Daily
exposure
to air
[ascorbic acid] / × 10
–
3
mol
dm
–
3
(i) Calculate the average rate of decrease in ascorbic acid concentration for the 24 h
sample over the period of 14 days, including units. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
12EP08

– 9 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
Turn over
(Question 2 continued)
(ii) The student’s hypothesis is: “A lower ascorbic acid concentration will be found in
juice exposed to the air for longer, due to oxidation of ascorbic acid by oxygen.”
Discuss whether or not the data support the hypothesis. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) State the implications of the results of the experiment for avoiding loss of vitamin
C in the storage of orange juice. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(e) Suggest an extension to the investigation that would generate further recommendations
for the storage of orange juice. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12EP09

– 10 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
3. A student investigates the effect of pressure on the volume of carbon dioxide gas released
from a carbonated drink poured into a flask.
To alter the pressure on the carbonated drink, masses are placed on top of the piston of the
gas syringe as shown.
masses
magnetic stirrer
fixed at set speed
carbonated
drink
magnet
piston of
gas syringe
The graph shows some of the data collected.
Experiment 2:
200
g attached
to piston
Experiment 1:
No added weights
to piston
0.0
10.0
20.0
30.0
40.0
Time / s
0.0 25.0 50.0
75.0
100.0
Volume of CO
2
/ cm
3
Experiment 3:
400
g attached
to piston
(a) Determine, by annotating the graph, the initial rate of release of CO
2
in Experiment 1 in
cm
3
s
−
1
. [2]
(This question continues on the following page)
12EP10

– 11 –
SPEC/4/CHEMI/HP1/ENG/TZ0/XX
0000 – 6102
(Question 3 continued)
(b) Estimate the time at which the piston would begin to move if a 600 g mass is used. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) Suggest two reasons why the volume of CO
2
collected in the gas syringe is smaller
when more masses are placed on top of the piston. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) Calculate the percentage decrease in the final volume of CO
2
per 100 g mass placed
on the piston, using the results of Experiment 1 and Experiment 3 at 100 s. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(e) Sketch and give a reason for a graph of the total mass of the apparatus in
Experiment 1 against time. [2]
Total mass
Time
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12EP11

Disclaimer:
Content used in IB assessments is taken from authentic, third-party sources. The views expressed within them belong to their
individual authors and/or publishers and do not necessarily reect the views of the IB.
References:
1(c) National Center for Biotechnology Information, 2020. PubChem Compound Summary for CID 24526, Chlorine
[online] Available at: <https://pubchem.ncbi.nlm.nih.gov/compound/Chlorine> [Accessed 23 September 2020].
1(e) Norlex, 2020. Chlorine for water disinfection. [online] Available at: <https://norlexpoolspa.com/guidance/about-the-
right-water-balance/safe> [Accessed 23 September 2020].
1(f)(ii) ResearchGate, 2015. Distributions of chloramines as a function of pH. [image online] Available at: <https://www.
researchgate.net/gure/Distribution-of-chloramines-as-a-function-of-pH_g8_273449675> [Accessed 23 September
2020].
12EP12

7 pages
Markscheme
Specimen paper
Chemistry
Higher level
Paper 1 – Section B
– 2 –
This markscheme is the property of the International Baccalaureate
and must not be reproduced or distributed to any other person
without the authorization of the IB Global Centre, Cardiff.

– 3 –
Question
Answers
Notes
Total
1.
(a)
Reactant: Cl
2
0
Products: HOCl +1
HCl -1
Award [2] for three correct.
Award [1] for any two correct.
2
1.
(b)
equilibrium shifts to the right/product AND HCl/HOCl/H
+
removed/neutralized
«by NaOH»
Accept any suitable equation to
illustrate the neutralization reaction.
1
1.
(c)
i
Any one of:
pressure not given
use of different units
OR
different ways of measuring
different precisions/significant figures
OR
uncertainties not given
1 max
1.
(c)
ii
0.177 «dm
3
» × 2.86 «g dm
-3
» / 0.506 «g»
OR
13
0.506«g»
70.9«g mol » 0.100«dm »
−
×
0.0714 «mol dm
-3
»
Award [2] for correct final answer.
Accept use of PV = nRT to calculate the
solubility. Using P = 100 kPa gives a
solubility of 0.0703 «mol dm
-3
».
2
1.
(c)
iii
«as temperature increases solubility decreases»
dissolution is exothermic «hence equilibrium shifts to reactants side at higher
temperatures»
OR
thermal energy overcomes intermolecular forces between chlorine and water
OR
negative entropy change «of dissolution» becomes more dominant at higher
temperatures
Accept “kinetic energy increases with
temperature «so more gas molecules
escape»”.
1

– 4 –
1.
(d)
Any one of:
toxic
gas
difficult to handle/store
1 max
1.
(e)
i
0 – 6
Accept any number or range below pH
6.5.
1
1.
(e)
ii
7.5
[OCl
-
] = [HOCl]
OR
[H
+
] = K
a
2
1.
(f)
i
NH
3
(aq) + 2HOCl(aq) NHCl
2
(aq) + 2H
2
O(l)
1
1.
(f)
ii
pH > 5/high
low concentration of Cl
2
/HOCl/NH
3
Do not accept general statements such as
“less urination in the pool”.
2
1.
(g)
Any two of
lower water evaporation
reduce energy consumption/less energy needed to heat the water
higher solubility of chlorine «so less chlorine lost»
Accept “less chlorine needed AND
fewer bacteria”.
2 max

– 5 –
Question
Answers
Notes
Total
2.
(a)
Any two for [1 max]
type of orange juice
temperature
light intensity
«initial» surface area
OR
«initial» volume AND flask
1 max
2.
(b)
i
Any one of:
to perform multiple titrations
too concentrated «so using too much iodine solution»
end-point colour easier to see
1 max
2.
(b)
ii
flask has to be opened to withdraw samples «so not 0 h»
OR
air was present in the flask at the start «so the ascorbic acid was
exposed to air»
1
2.
(b)
iii
titrate only once after two weeks
OR
fill flask with nitrogen/argon/inert gas
OR
withdraw samples with syringe «without opening flask»
1
2.
(c)
3
3
0.1 cm
« 100 » 0.7 «%»
15.0 cm
×=
1
2.
(d)
i
33 33
4
(5.3 10 mol dm 1.1 10 mol dm )
« »3.0 10
14d
−− −−
−
× −×
= ×
mol dm
-3
d
-1
Accept values in the range 2.9 × 10
-4
–
3.1 × 10
-4
.
Accept values converted to other units,
such as
3.4 × 10
–9
– 3.6 × 10
–9
mol dm
–3
s
–1
or 0.29–0.31 mmol dm
–3
d
–1
.
2

– 6 –
2.
(d)
ii
«support» longer daily exposure leads to lower concentration of ascorbic acid
OR
0 h decreases the least
«doesn’t support» no direct evidence of oxidation by oxygen
2
2.
(d)
iii
should be stored in sealed container
OR
should be consumed in a few days after opening
1
2.
(e)
Any one of:
effect of temperature/light AND would show the value of refrigeration/darkness
effect of preservative
compare types of orange juice «e.g. fresh, from concentrate, etc.»
1 max

– 7 –
Question
Answers
Notes
Total
3.
(a)
tangent drawn at t = 0
«
3
40.0 cm
35.0 s
=» 1.14 «cm
3
s
-
1
»
Accept values in the range
1.04 – 1.24 cm
3
s
-1
.
2
3.
(b)
any value between 35 and 50 s
Only a simple estimation is required.
1
3.
(c)
Any two:
drink releases gas more slowly at higher pressure
gas occupies lower volume at higher pressure
gas is more soluble «in carbonated drink» at higher pressure
2 max
3.
(d)
«
33
3
33.0 cm 11.5 cm– 100
4
33.0 cm
×=
» 16.3 «% per 100 g»
Accept values in the range 15.8 – 16.7
«% per 100 g».
1
3.
(e)
horizontal line
closed system
2

Candidate session number
© International Baccalaureate Organization 2023
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
27 pages
Specimen paper
2 hours 30 minutes
Chemistry
Higher level
Paper 2
Instructions to candidates
y Write your session number in the boxes above.
y Do not open this examination paper until instructed to do so.
y Answer all questions.
y Answers must be written within the answer boxes provided.
y A calculator is required for this paper.
y A clean copy of the chemistry data booklet is required for this paper.
y The maximum mark for this examination paper is [90 marks].
28EP01

– 2 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Answer all questions. Answers must be written within the answer boxes provided.
1. A monoprotic acid, HX, is found to have the following composition by mass:
C = 39.99 % H = 6.73 % O = 53.28 %
(a) Determine the empirical formula of the compound HX. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) 25.00 cm
3
of a solution, containing 1.51 g of HX is titrated with a 0.750 mol dm
-
3
solution of NaOH (aq). The HX (aq) solution is exactly neutralized by 22.30 cm
3
of the
NaOH (aq) solution. Determine the molar mass (M) of HX. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) State the molecular formula of HX. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) HX reacts with aqueous sodium hydroxide according to the equation:
HX (aq) +NaOH(aq)→NaX(aq)+ H
2
O (l)
Identify a functional group present in HX. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP02

– 3 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 1 continued)
(e) The IR spectrum of HX is shown.
IR spectrum
3000 2500
Wavenumber / cm
–1
Transmittance / %
2000 1500 1000 500
0
4000
100
20
0
40
60
80
3500
Y
Z
(i) Identify the functional groups responsible for the absorption bands shown at Y
and Z using section 20 of the data booklet. [1]
Y: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Z: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Draw a structural formula of HX that is consistent with all the evidence. [1]
28EP03

– 4 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
2. Scientific models are used to explain the structure of matter.
(a) An α-particle is a helium-4 nucleus. In an experiment, α-particles are accelerated
towards a thin sheet of gold and their resulting paths are detected, giving evidence of
the positive charge of the nucleus.
Thin sheet of gold
Path
II
α
-particles
Accelerated
Path
I
Angle of detection θ increase from 0° to 180°
θ
The number of α-particles detected at different angles of deflection θ are shown.
180° 180°90° 0° 90°
Path
II Path II
Path I
θ
Number of
α-particles detected
Key:
(This question continues on the following page)
28EP04
– 5 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 2 continued)
(i) Explain why some α-particles follow path II, rebounding from the gold sheet. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Most of the α-particles follow path I and pass straight through
undeflected (θ = 0°). Suggest a conclusion that can be made about the structure
of the atom based on this evidence. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP05

– 6 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
(Question 2 continued)
(b) Helium was first identified by analysing spectra of solar radiation.
(i) Outline the appearance of the emission spectrum of helium. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Emission spectra of one-electron systems can be explained using a model with
the electron attracted to the nucleus by an electrostatic force.
This model predicts that the electron occupies discrete energy levels. Some energy
levels for the He
+
ion are shown.
0
n
∞
n 3
n 2
n 1
328
582
1310
5250
Energy / kJ mol
1
(ii) Explain how the frequencies observed in emission spectra support the idea of the
electron occupying discrete energy levels. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP06

– 7 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 2 continued)
(iii) Deduce the ionization energy of the He
+
ion from the energy levels shown. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iv) Suggest two reasons why the ionization energy of the hydrogen atom is
significantly smaller than the ionization energy of the He
+
ion. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(v) Suggest why the model outlined in (b)(ii) can predict the emission spectrum of
He
+
but not He. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) Outline why models of the atom have evolved over time. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
28EP07
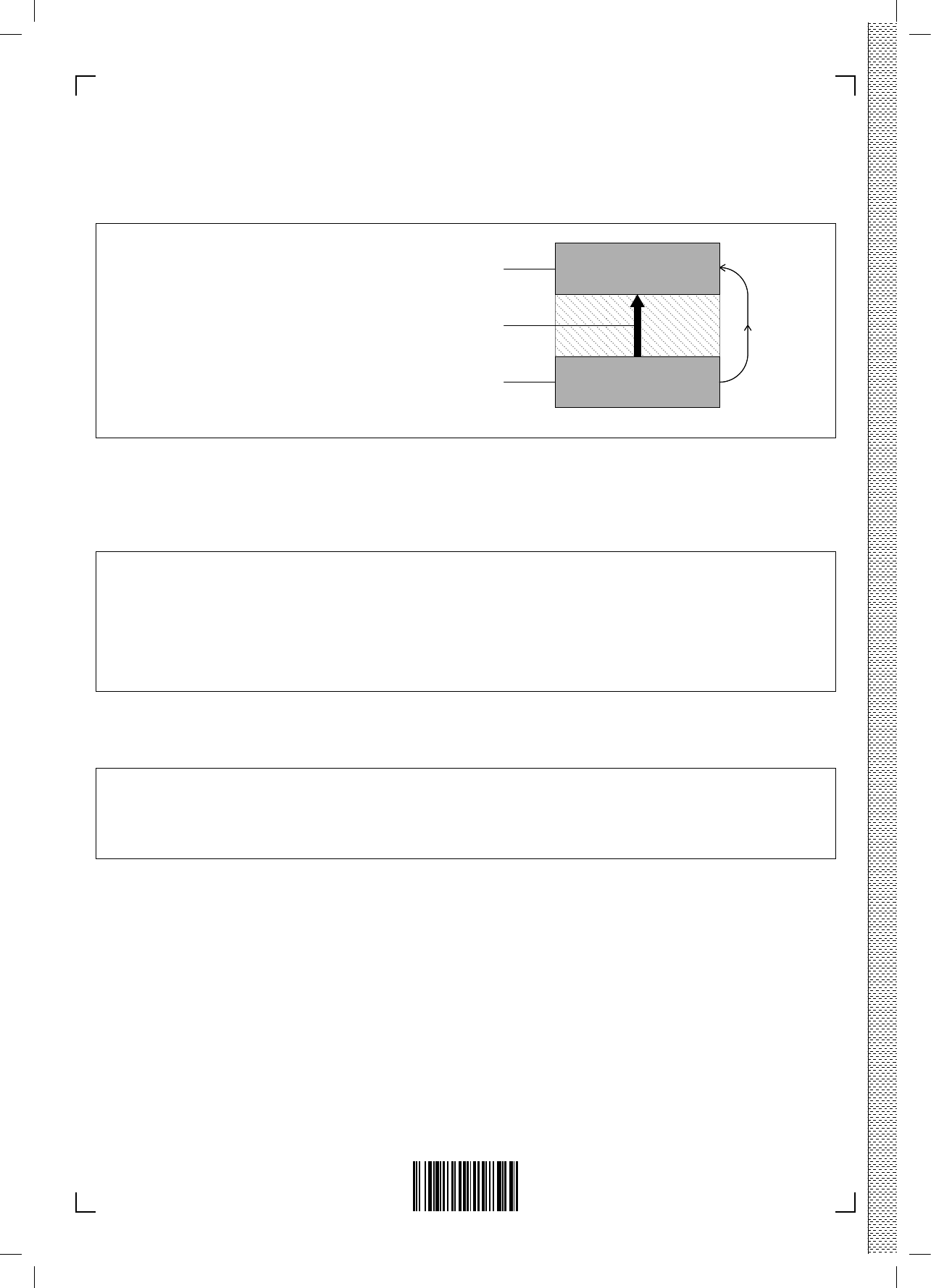
– 8 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
3. The development of the lithium-ion battery won the 2019 chemistry Nobel Prize.
(a) The diagram represents a cell in such a battery delivering a current. Complete the
half-equations on the diagram and identify the species moving between the electrodes. [3]
Li(CoO
2
)
2
2LiCoO
2
Species moving:
Cathode
(LiCoO
2
)
Anode
(Li in graphite lattice)
Electron flow
(b) The discharge of the lithium-ion battery is a spontaneous chemical reaction producing
a potential difference and an increase in temperature.
(i) Deduce the signs of the following: [1]
ΔH
discharge
: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
E
cell
: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
ΔG
discharge
: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) The lithium-ion battery can be recharged by reversing the reactions at each
electrode. Compare the absolute value of ΔG
recharge
and ΔG
discharge
. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP08

– 9 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 3 continued)
(c) In a simulation, equal masses of potassium and lithium are added to water and the time
taken for the metals to fully react is recorded. Five different increasing masses of each
metal are used, and the reaction is timed.
(i) Sketch the graphs on the axes to show the expected results of this experiment. [2]
Mass of potassium / g
Time for metal to react completely
Mass of lithium / g
Time for metal to react completely
(ii) Suggest a reason why comparing the time for complete reaction of equal masses
is not a valid measure of reactivity. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Lithium carbide, Li
2
C
2
, is one of many compounds of lithium and carbon.
Determine the percentage covalent character and bonding type in this compound
by using sections 9 and 17 of the data booklet. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on page 11)
28EP09

Please do not write on this page.
Answers written on this page
will not be marked.
– 10 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
28EP10

– 11 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 3 continued)
(iv) Calculate the Gibbs energy of formation, ΔG
f
Ö
, in kJ mol
-
1
, for Li
2
C
2
at 298.15 K.
Use the data provided and section 1 of the data booklet. [1]
ΔH
f
Ö
Li
2
C
2 solid
= -62
kJ mol
-
1
ΔS
f
Ö
Li
2
C
2 solid
= -11
J mol
-
1
K
-
1
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(v) Draw the Lewis formula of the anion in the salt Li
2
C
2
. [2]
(vi) State the type of hybridization shown by the carbon atoms in the anion. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
28EP11

Please do not write on this page.
Answers written on this page
will not be marked.
– 12 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
28EP12

– 13 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
4. Hydrochloric acid is an important chemical reactant and industrial chemical.
(a) A pH probe is placed in a small volume of 0.10 mol dm
-
3
solution of hydrochloric acid.
The pH is recorded while a steady stream of distilled water is added to the acid at
constant temperature.
(i) On the axes, sketch the graph of pH against volume of water added. [3]
Volume of H
2
O added
pH
0
4
6
8
10
12
14
2
(ii) The experiment is repeated using 0.10 mol dm
-
3
ethanoic acid at the same
temperature. Calculate the initial pH of the ethanoic acid. [2]
pK
a
ethanoic acid = 4.76
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP13

– 14 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
(Question 4 continued)
(b) Chloride ions can form complex ions with some transition metals. The formulas and
colours of three compounds of cobalt are:
Compound Colour
[Co(NH
3
)
6
]Cl
3
orange–yellow
[Co(NH
3
)
5
Cl]Cl
2
purple
[Co(NH
3
)
4
Cl
2
]Cl
green
(i) Deduce the oxidation state of cobalt in [Co(NH
3
)
5
Cl]Cl
2
and the charge of the
complex ion. [2]
Oxidation state: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Charge of complex ion: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Describe the bonding of chloride ions in [Co(NH
3
)
4
Cl
2
]Cl. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Explain why these complex ions are coloured. [3]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP14

– 15 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 4 continued)
(iv) State and explain, in which of the complex ions, the electron transitions
responsible for the colour require the highest energy. Use the colour wheel and
the electromagnetic spectrum in sections 5 and 15 of the data booklet. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
28EP15

Please do not write on this page.
Answers written on this page
will not be marked.
– 16 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
28EP16

– 17 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
5. Heptadecane, C
17
H
36
, can be extracted from crude oil or cactus plants.
(a) Write an equation for the complete combustion of C
17
H
36
. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) The enthalpy of combustion of C
17
H
36
is -11 350 kJ mol
-
1
.
(i) Calculate the maximum energy produced when 2.00 g of C
17
H
36
is combusted. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Determine the maximum temperature change when 500.0 cm
3
of water is heated
by a 2.00 g sample of C
17
H
36
. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Outline two assumptions made in the calculation in (b)(ii). [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP17

– 18 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
(Question 5 continued)
(c) Explain why biofuels contribute less to climate change than fossil fuels. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) Heptadecane can be broken down into smaller molecules. Consider the reaction:
C
17
H
36
(g)→2C
2
H
4
(g) + C
13
H
28
(g)
(i) Determine the standard enthalpy change, ΔH
Ö
, for the reaction stated, using
section 12 of the data booklet. [3]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Determine the enthalpy change of reaction. Use the data provided and section 13
of the data booklet. [2]
ΔH
f
(C
17
H
36
) = -393.9 kJ mol
-
1
ΔH
f
(C
13
H
28
) = -311.5 kJ mol
-
1
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP18

– 19 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 5 continued)
(iii) Comment on the difference between the two values calculated in (d)(i) and (d)(ii). [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iv) Predict the sign of the entropy change of the reaction, giving a reason. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(v) Discuss, with reference to (d)(ii) and (d)(iv), how temperature affects the
spontaneity of the reaction. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(e) Ethene can be converted to ethanol in one reaction. State the equation for this reaction. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP19

– 20 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
(Question 5 continued)
(f) Ethanol reacts with oleic acid to produce ethyl oleate.
C
17
H
33
COOH (l) + CH
3
CH
2
OH (l) C
17
H
33
COOCH
2
CH
3
(l) + X (l)
(i) Identify the type of reaction and the side product X (l). [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Calculate the atom economy of the reaction. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Discuss why the atom economy of a reaction is an important consideration when
evaluating the impact of a reaction in an industrial process. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iv) Deduce the equilibrium constant expression, K
c
, for the reaction. Assume that the
reaction is homogeneous. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP20

– 21 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 5 continued)
(g) The equilibrium constant, K
c
, is 9.3 10
-
5
at 75 °C.
(i) Determine the amount of ethyl oleate present in the reaction mixture at
equilibrium when 0.10 mol dm
-
3
of oleic acid reacts with 0.10 mol dm
-
3
of ethanol
at 75 °C and state any assumptions you have made in your calculation. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) State one method of increasing the yield. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
28EP21

Please do not write on this page.
Answers written on this page
will not be marked.
– 22 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
28EP22

– 23 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
6. Halogens are important reactants in the laboratory and in the environment.
(a) (i) Write an equation for the homolytic fission of chlorine under UV light, showing the
movement of electrons. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Under different conditions, chlorine molecules can break down by
heterolytic fission. Write an equation showing the movement of electrons. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Identify, giving a reason, which one of the three species produced in (a)(i) and
(a)(ii) is an electrophile. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iv) Draw the full structural formula of the most stable carbocation formed in the
reaction between hydrogen chloride and propene. [1]
(v) Outline why this intermediate is the most stable. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP23

– 24 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
(Question 6 continued)
(b) The graph shows the boiling points of the first five straight-chain primary alcohols and
fluoroalkanes.
Relative formula mass
Primary alcohols
Primary fluoroalkanes
Boiling point / °C
150
50
0
50
100
150
20 30 40 50 60 70 80 90 10010
100
Key:
(i) Outline why the alcohols have higher boiling points than fluoroalkanes of similar
relative formula mass. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Explain the general trend in the boiling points shown for the alcohols. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
28EP24

– 25 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
Turn over
(Question 6 continued)
(iii) Predict, giving a reason, how the boiling points of branched fluoroalkanes
compare to their straight-chain isomers. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iv) Explain why primary bromoalkanes have higher boiling points compared to the
corresponding fluoroalkanes. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) (i) Draw the structures of 2-bromobutane and its tertiary isomer. [2]
(ii) One of the isomers in (c)(i) exists as enantiomers. Draw their
stereochemical formulas. [1]
(This question continues on the following page)
28EP25

– 26 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
(Question 6 continued)
(iii) The IR and
1
H NMR spectra of 2-bromobutane are shown. Circle the regions
which indicate the presence of the bromine atoms. Use sections 20 and 21 of
the data booklet. [2]
2-Bromobutane infrared spectrum
3000 2500
Wavenumber / cm
–1
Transmittance / %
2000 1500 1000 500
0
4000
100
20
0
40
60
80
3500
1
H NMR spectrum
6 5
ppm
4 3 2 1
0
8910
11
7
(This question continues on the following page)
28EP26

– 27 –
SPEC/4/CHEMI/HP2/ENG/TZ0/XX
0000 – 6103
(Question 6 continued)
(iv) Explain whether the IR and
1
H NMR spectra can be used to distinguish the
tertiary isomer from 2-bromobutane. [2]
IR: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1
H NMR: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
28EP27

Disclaimer:
Content used in IB assessments is taken from authentic, third-party sources. The views expressed within them belong to their
individualauthorsand/orpublishersanddonotnecessarilyreecttheviewsoftheIB.
References:
6. (c)(iii) [2-Bromobutane infrared spectrum] NIST, 1960. Butane, 2-bromo-. NIST Chemistry WebBook. [online]
Available at: <https://webbook.nist.gov/cgi/cbook.cgi?ID=C78762&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC>
[Accessed 1 February 2021].
[
1
H NMR spectrum] ChemicalBook, n.d. 2-Bromobutane (78-76-2) 1H NMR. [CAS DataBase List>78-76-2More
Spectrum> 2-Bromobutane (78-76-2) 1H NMR] ChemicalBook [online]
Available through: <https://www.chemicalbook.com/SpectrumEN_78-76-2_1HNMR.htm>
[Accessed 1 February 2021].
28EP28

17 pages
Markscheme
Specimen paper
Chemistry
Higher level
Paper 2
– 2 –
This markscheme is the property of the International Baccalaureate
and must not be reproduced or distributed to any other person
without the authorization of the IB Global Centre, Cardiff.

– 3 –
Question
Answers
Notes
Total
1. (a)
n
C
= «
1
39.99g
12.01gmol
−
=
» 3.33 «mol»
n
H
= «
1
6.73g
1.01gmol
−
=
» 6.66 «mol»
n
O
= «
1
53.28g
16.00gmol
−
=
=» 3.33 «mol»
CH
2
O
2
1. (b)
n
HX
(= nNaOH =) 0.750 «mol dm
-3
» × 0.02230 «dm
3
» / 0.0167 «mol»
M
HX
= (
1.51 g
0.0167mol
=
) 90.4 g mol
-1
Accept 90.3 «g mol
–1
». 2
1. (c) C
3
H
6
O
3
Accept consistent feasible structural
formula.
1
1.
(d)
carboxyl/COOH
Do not accept “carbonyl/C=O”.
1
1. (e) (i)
Y: O-H/hydroxyl «with hydrogen bonding»
AND
Z: C=O/carbonyl «in carboxylic acid»
1
1. (e) (ii)
OR
HO–CH
2
–CH
2
–COOH
1

– 4 –
Question
Answers
Notes
Total
2.
(a)
(i)
repelled by «hitting/close contact with» gold nucleus
1
2. (a) (ii)
atom is mainly empty space/vacuum
OR
nucleus is very small «compared to the size of the atom»
1
2.
(b)
(i)
discrete/series of lines of different frequency/wavelength
1
2. (b) (ii)
energy of photon relates to a frequency «in the spectrum»
energy of photon depends on difference in energy levels
2
2.
(b)
(iii)
5250 «kJ mol
-1
»
1
2. (b) (iv)
H half/lower nuclear charge/number of protons
H larger/double radius
2
2.
(b)
(v)
electron-electron interactions «need to be taken in account»
1
2. (c)
Any one of:
new evidence
new technology
developments in related models
models incomplete/failed to account for all observations
1

– 5 –
Question
Answers
Notes
Total
3. (a)
3
3. (b) (i)
ΔH
discharge
: negative AND
E
cell
: positive AND
ΔG
discharge
: negative
1
3.
(b)
(ii)
ΔG
recharge
> ΔG
discharge
1
3. (c) (i)
separate curves/lines for Li and K sketched AND both increasing
steeper gradient for Li
OR
curve/line for Li higher
2
3.
(c)
(ii)
equal masses «of different substances» do not contain equal amounts/moles
1
3. (c) (iii)
«Avg electronegativity = 1.8
Δ electronegativity = 1.6»
% covalent character = 45-55
ionic
2
3. (c) (iv)
«∆G
⦵
= ∆H
⦵
− T∆S
⦵
= -62 – 298 × -0.011 =»
-59 «kJ mol
-1
»
1

– 6 –
Question
Answers
Notes
Total
3. (c) (v)
[:C≡C:]
2-
2- charge
:C≡C:
2
3.
(c)
(vi)
sp
1

– 7 –
Question
Answers
Notes
Total
4. (a) (i)
start at pH = 1
curve with decreasing gradient
must finish below pH = 7
3
4. (a) (ii)
K
a
=
−+
3
3
[H ][CH COO ]
[CH COOH]
OR
K
a
=
+ 2
3
[H ]
[CH COOH]
OR
pK
a
= 2pH – 1
OR
pH =
a
p1
2
K
+
=
2.88
2

– 8 –
Question
Answers
Notes
Total
4. (b) (i)
Oxidation state: +3
Charge of complex ion: 2+
2
4. (b) (ii)
two chlorides covalently bonded/coordination bond to cobalt ion
one chloride ionically bonded to complex ion
2
4. (b) (iii)
3d sub-level split in presence of ligands
light is absorbed when electrons promoted between split-levels
colour seen is complementary to the light absorbed
3
4. (b) (iv)
complex [Co(NH
3
)
6
] Cl
3
absorbs blue-purple light/wavelength 424 nm/shortest
wavelength/highest energy
2

– 9 –
Question
Answers
Notes
Total
5.
(a)
C
17
H
36
(l) + 26O
2
(g) → 17CO
2
(g) + 18H
2
O(l)
1
5. (b) (i)
nC
17
H
36
= «
− −−
= =
× +×
1 11
2.00 g 2.00 g
[(17 12.01g mol ) (36 1.01g mol )] 240.53 g mol
»
0.008315/0.00831 «mol»
« energy = 11350 kJ mol
-1
× 0.008315 mol = » 94.4 «kJ»
2
5. (b) (ii)
94 400 = 500.0 g × 4.18 J g
−1
K
−1
× ∆T
∆T = 45.2«K»
2
5. (b) (iii)
Any two:
water does not evaporate
heat is not lost to the surroundings
OR
all heat is transferred to the water
density of water is 1 g cm
-3
water is pure
complete combustion
2 max

– 10 –
Question
Answers
Notes
Total
5. (c)
CO
2
consumed while plant is growing «and later released when biofuel is
combusted»
OR
photosynthesis uses up CO
2
«later released when biofuel is combusted»
1
5. (d) (i)
bonds broken: 4(C ̶̶ C ) / 4 × 346
bonds formed: 2(C=C) / 2 × 614
ΔH
ϴ
= «4 × 346 kJ – 2 × 614 kJ / 1384 kJ – 1228 kJ =»
ΔH
ϴ
= «+»156 «kJ»
Award [3] for correct final answer. 3
5. (d) (ii)
«∆H
= Σ∆H
f
(products) - Σ∆H
f
(reactants)»
∆H
= (-311.5 + 2(52.0)) – (-393.9)
∆H
= «+»186.4 «kJ mol
-1
»
2
5. (d) (iii)
(d)(ii) more accurate than (d)(i) as bond energies are average values
OR
(d)(ii) more accurate than (d)(i) as not specific to bonds in the reaction
1
5.
(d)
(iv)
positive AND increase in number of moles/molecules «of gas»
1
5. (d) (v)
ΔH> 0 «and ΔS > 0» AND reaction spontaneous if ΔG «= ΔH – TΔS» < 0
at high«er» T reaction «more» spontaneous/ΔG «more» negative
2
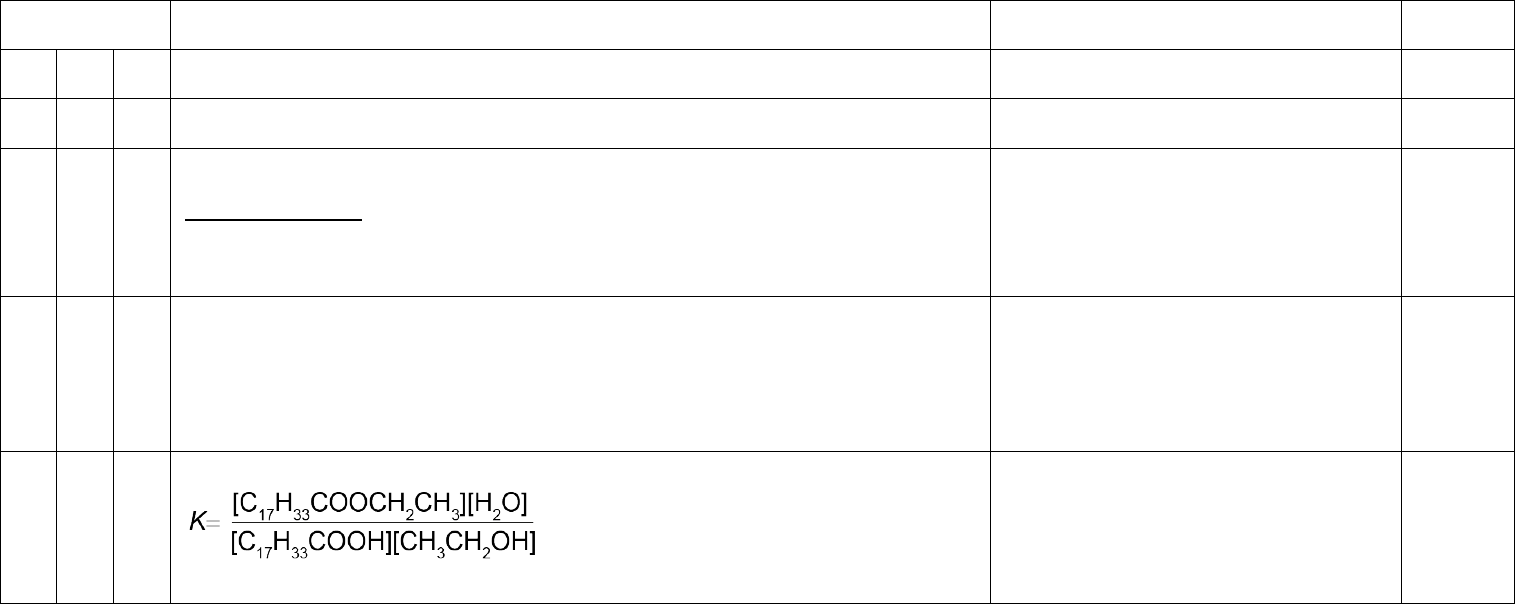
– 11 –
Question
Answers
Notes
Total
5.
(e)
C
2
H
4
(g) + H
2
O(g) → C
2
H
5
OH(g)
1
5.
(f)
(i)
condensation/esterification AND H
2
O / water
1
5. (f) (ii)
«20(12.01) + 38(1.01) + 2(16.00) = 310.58
×
=
+
100 310.58
(310.58 18.02)
»
94.5 %
1
5. (f) (iii)
Any two of:
sustainable development
more economical/efficient
better use of natural resources
reduces waste
2 max
5. (f) (iv)
Accept expressions with [X(l)] instead
of [H
2
O].
1

– 12 –
Question
Answers
Notes
Total
5. (g) (i)
K small AND [reactants]
eqm
= [reactants]
0
/0.10
n
oleate
= « (0.10 × 0.10 × 9.3×10
-5
)
½
=» 9.6×10
-4
«mol»
2
5. (g) (ii)
remove water
OR
add more oleic acid
OR
add more ethanol
1

– 13 –
Question
Answers
Notes
Total
6. (a) (i)
→ 2 Cl•
2 Cl•
single-barbed/fish-hooks
Accept chlorine atoms. 2
6. (a) (ii)
→
full/double-barbed arrow AND charges on both ions are required for mark.
1
6.
(a)
(iii)
Cl
+
AND can accept a pair of electrons to form a new bond
1
6. (a) (iv)
1
6.
(a)
(v)
positive inductive effects from two -CH
3
/methyl groups
1

– 14 –
Question
Answers
Notes
Total
6.
(b)
(i)
hydrogen bonding stronger than dipole-dipole
1
6. (b) (ii)
«increase because» stronger London/dispersion forces
more electrons
OR
surface contact
2
6. (b) (iii)
lower AND more compact shape reduces contact area/London/
dispersion forces
1
6.
(b)
(iv)
more electrons increase London/dispersion forces
1

– 15 –
Question
Answers
Notes
Total
6. (c) (i)
2
6. (c) (ii)
1

– 16 –
Question
Answers
Notes
Total
6. (c) (iii)
2
– 17 –
Question
Answers
Notes
Total
6. (c) (iv)
IR: no AND same bonds
1
H NMR: yes AND tertiary «isomer» one signal
Accept "yes AND difference in
"fingerprint" region/1500–500 cm
–1
/fewer
peaks in tertiary isomer «due to more
symmetrical molecule».
2

© International Baccalaureate Organization 2023
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
11 pages
Specimen paper
1 hour 30 minutes [Paper 1A and Paper 1B]
Chemistry
Standard level
Paper 1A
Instructions to candidates
y Do not open this examination paper until instructed to do so.
y Answer all questions.
y For each question, choose the answer you consider to be the best and indicate your choice on
the answer sheet provided.
y A calculator is required for this paper.
y A clean copy of the chemistry data booklet is required for this paper.
y The maximum mark for paper 1A is [30 marks].
y The maximum mark for paper 1A and paper 1B is [55 marks].

– 2 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
Section A
1. Which technique is used to purify a solid obtained from a chemical reaction?
A. distillation
B. evaporation
C. recrystallization
D. filtration
2. Ice containing only the isotope
2
H sinks and does not melt when dropped into ordinary
distilled water maintained at 3
C.
Which statement is correct?
A. The isotope
2
H has a high natural abundance.
B.
2
H
2
O (s) has a higher melting point than normal ice.
C.
2
H
2
O (s) has a lower density than normal ice-cold water.
D.
2
H
2
O has different chemical properties from normal water.
3. Which electron transition in the hydrogen atom emits radiation with the highest energy?
A. n = 1 to n = 2
B. n = 2 to n = 3
C. n = 2 to n = 1
D. n = 3 to n = 2
4. A container holds 30 g of argon and 60 g of neon.
What is the ratio of number of atoms of argon to number of atoms of neon in the container?
A. 0.25
B. 0.50
C. 2.0
D. 4.0

– 3 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
Turn over
5. A gas storage tank of fixed volume V contains N molecules of an ideal gas at 300 K with a
pressure of 40 kPa.
N
4
molecules are removed, and the temperature is changed to 450 K.
What is the new pressure of the gas in kPa?
A. 15
B. 30
C. 45
D. 60
6. What is the formula of the compound formed between magnesium ions and
hydrogencarbonate ions?
A. MgHCO
3
B. Mg(HCO
3
)
2
C. Mg(HCO
3
)
3
D. Mg
3
(HCO
3
)
2
7. Which species contains a coordination bond?
A. CO
2
B. HCN
C. NO
2
+
D. NO
3
−
8. Which properties depend on the movement of the delocalized electrons in a metal?
I. Electrical conductivity
II. Thermal conductivity
III. Density
A. I and II only
B. I and III only
C. II and III only
D. I, II and III

– 4 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
9. Which substance, made from two elements with electronegativities E
X
and E
Y
, is an alloy?
Average electronegativity
EE
XY
++
2
Electronegativity difference
EE
XY
−
A. 2.5 2.5
B.
2.5 1.0
C. 3.5 0.2
D. 1.2 0.2
10. Which structure shows the repeating unit of the polymer formed by but-1-ene?
A.
C C C
H
H
H
H
H
CH
3
B.
C C
H
CH
3
H
CH
3
C.
C C
CH
3
H
H
CH
3
D.
C C
H
H
H
CH
2
CH
3
11. What is the explanation for the malleability of metals?
A. The bonds are strong.
B. The bonds are weak.
C. The bonds involve free electrons.
D. The bonds do not have a specific direction.

– 5 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
Turn over
12. Which functional groups are present in this molecule?
A. amino, alkoxy, ester
B. ether, carboxyl, amino
C. carboxyl, alkoxy, ester
D. ester, amino, carboxyl
13. In which block of the periodic table would element 119 be placed, if it is found in the future?
A. s
B. p
C. d
D. f

– 6 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
14. Which is a correct alternative representation of this molecule?
C
C
C
H
H
H
H
H
H
C
Cl
Cl
A. C
3
H
6
Cl
2
B.
Cl
Cl
C. 2,2-dichlorobut-1-ene
D. CH
3
CHClC(Cl)=CH
2
15. The block structure of the periodic table groups elements according to which characteristic?
A. atomic number
B. atomic mass
C. electron configuration
D. reactivity
16. Which set of conditions describe a reaction in which the reactants are more stable than
the products?
A. endothermic and ∆H negative
B. endothermic and ∆H positive
C. exothermic and ∆H negative
D. exothermic and ∆H positive

– 7 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
Turn over
17. Which enthalpy changes can be calculated using only bond enthalpy data?
I. N
2
(g) + 2H
2
(g) → N
2
H
4
(g)
II. CH
4
(g) + 2O
2
(g) → 2H
2
O (l) + CO
2
(g)
III. H
2
(g) + Cl
2
(g) → 2HCl (g)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
18. Which is a renewable energy source?
A. natural gas
B. uranium
C. coal
D. wood
19. What is the amount, in mol, of H
2
O produced for a reaction between 10.0 mol of C
2
H
3
Cl and
10.0 mol of O
2
if the yield is 90 %?
2C
2
H
3
Cl (g) + 5O
2
(g) → 4CO
2
(g) + 2H
2
O (g) + 2HCl (g)
A. 3.60
B. 4.00
C. 9.00
D. 10.00
20. The complete combustion of 20.0 cm
3
of a gaseous hydrocarbon, C
x
H
y
, produces 80.0 cm
3
of gaseous products. This volume reduces to 40.0 cm
3
when the water vapour present
condenses. All volumes are measured at the same temperature and pressure.
What is the molecular formula of the hydrocarbon?
A. CH
4
B. C
2
H
2
C. C
2
H
4
D. C
3
H
6

– 8 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
21. Large deposits of methane hydrate, CH
4
⋅6H
2
O (M
r
= 124), have been discovered under the
ocean floor. What mass of carbon dioxide would be produced by the complete combustion of
12.4 g of the methane hydrate?
A. 4.40 g
B. 26.4 g
C. 34.1 g
D. 44.0 g
22. The diagram shows the energy profile of a reaction.
Reactants
Products
Reaction coordinate
Potential energy
X
Y
Z
Which combination is correct?
Activation energy of
forward reaction
Activation energy of
reverse reaction
A. X Z
B.
Y − X Y − Z
C. Y Y
D.
Y − X Z − X

– 9 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
Turn over
23. What is the main reason for an increase in rate of reaction when the temperature is raised?
A. A greater proportion of collisions are successful.
B. Particles collide more frequently.
C. The bonds in the reactants are weakened.
D. The activation energy of the reaction decreases.
24. What is the equilibrium constant expression for the following reaction?
2SO
3
(g) 2SO
2
(g) + O
2
(g)
A.
[SO][O ]
[SO]
2
2
2
3
2
B.
[SO] [O ]
[SO]
2
2
2
3
2
+
C.
[SO]
[SO][O ]
3
2
2
2
2
D.
2[SO ][O ]
2[SO ]
22
3
25. Which reactions involve the transfer of a proton?
I. 2HCl (aq) + Mg (s) → MgCl
2
(aq) + H
2
(g)
II. 2HCl (aq) + MgO (s) → MgCl
2
(aq) + H
2
O (l)
III. 2HCl (aq) + MgCO
3
(s) → MgCl
2
(aq) + H
2
O (l) + CO
2
(g)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III

– 10 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
26. The overall reaction occurring at the electrodes of a rechargeable metal hydride battery can
be summarized as:
MH + NiO(OH) M + Ni(OH)
2
Which statement is correct?
A. The oxidation state of Ni does not change.
B. M is oxidized by loss of hydrogen.
C. The oxidation state of one H atom changes from −1 to +1.
D. The oxidation state of one O atom changes from −1 to −2.
27. In a redox titration, manganate(VII) ions are reduced to manganese(II) ions and iron(II) ions
are oxidized to iron(III) ions.
MnO
4
−
(aq) reduced to Mn
2
+
(aq)
Fe
2
+
(aq) oxidized to Fe
3
+
(aq)
What volume, in cm
3
, of 0.1 mol dm
−
3
MnO
4
−
(aq) is required to reach the equivalence point in
the titration of 20.00 cm
3
of 0.1 mol dm
−
3
Fe
2
+
(aq)?
A. 2.00
B. 4.00
C. 20.00
D. 100.00
28. What is the organic product of the reaction of 1-chloropentane with aqueous sodium hydroxide?
A. pentan-1-ol
B. 1-chloropentan-1-ol
C. 1-chloropent-1-ene
D. 1-chloropent-2-ene

– 11 –
SPEC/4/CHEMI/SPM/ENG/TZ0/XX
0000 – 6104
29. Which statements explain the following reactions occurring in the upper atmosphere?
Chlorofluorocarbon (CFC) compounds
break down to produce chlorine radicals
but usually not fluorine radicals.
A single chlorine radical breaks down
many ozone, O
3
, molecules.
A.
C–Cl bond is stronger than C–F bond
chain propagation steps produce more
radicals
B.
C–F bond is stronger than C–Cl bond
chain termination steps cause chlorine
radicals to reform chlorine molecules
C.
C–Cl bond is stronger than C–F bond
chain termination steps cause chlorine
radicals to reform chlorine molecules
D.
C–F bond is stronger than C–Cl bond
chain propagation steps produce more
radicals
30. Which species can act as an electrophile?
A. CH
4
B. Cl
2
C. Cl
−
D. OH
−

2 pages
Markscheme
Specimen paper
Chemistry
Standard level
Paper 1 – Section A

– 2 –
1. C 16. B 31. – 46. –
2. B 17. B 32. – 47. –
3. C 18. D 33. – 48. –
4. A 19. A 34. – 49. –
5. C 20. C 35. – 50. –
6. B 21. A 36. – 51. –
7. D 22. B 37. – 52. –
8. A 23. A 38. – 53. –
9. D 24. A 39. – 54. –
10. D 25. C 40. – 55. –
11. D 26. C 41. – 56. –
12. A 27. B 42. – 57. –
13. A 28. A 43. – 58. –
14. D 29. D 44. – 59. –
15. C 30. B 45. – 60. –

Candidate session number
© International Baccalaureate Organization 2023
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
9 pages
Specimen paper
1 hour 30 minutes [Paper 1A and Paper 1B]
Chemistry
Standard level
Paper 1B
Instructions to candidates
y Write your session number in the boxes above.
y Do not open this examination paper until instructed to do so.
y Answer all questions.
y Answers must be written within the answer boxes provided.
y A calculator is required for this paper.
y A clean copy of the chemistry data booklet is required for this paper.
y The maximum mark for paper 1B is [25 marks].
y The maximum mark for paper 1A and paper 1B is [55 marks].
12EP01

Please do not write on this page.
Answers written on this page
will not be marked.
– 2 –
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
12EP02

– 3 –
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
Turn over
Section B
Answer all questions. Answers must be written within the answer boxes provided.
1. Hypochlorous acid, HOCl, is a sterilizing agent used in swimming pools and is produced
when chlorine reacts with water.
Cl
2
(aq) + H
2
O (l) HOCl (aq) + HCl (aq)
(a) Deduce the oxidation states of chlorine in the reactants and products. [2]
Reactant: Cl
2
:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Products: HOCl:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
HCl:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Explain why more chlorine reacts with water when NaOH (aq) is added. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) Researchers studying the solubility of chlorine in pure water at different temperatures
compile the following data from different sources.
Source
Temperature /
C
Solubility of chlorine
A 0 1.46 g per 100 cm
3
B 10 310 cm
3
per 100 cm
3
C 20 0.70 g per 100 cm
3
D 25 6300 mg per 1000 cm
3
E 30 177 cm
3
per 100 cm
3
F 30 0.57 g per 100 cm
3
(This question continues on the following page)
12EP03

– 4 –
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
(Question 1 continued)
(i) Identify a problem in comparing the data from different sources. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) The units of solubility are converted to mol dm
−
3
.
Source
Temperature /
C
Solubility of chlorine Solubility of chlorine
/ mol dm
−
−
3
A 0 1.46 g per 100 cm
3
0.206
B 10 310 cm
3
per 100 cm
3
0.13
C 20 0.70 g per 100 cm
3
0.099
D 25 6300 mg per 1000 cm
3
0.089
E 30 177 cm
3
per 100 cm
3
F 30 0.57 g per 100 cm
3
0.080
Complete the table by calculating the value for source E.
Assume the density of chlorine is 2.86 g dm
−
3
at 30
C. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Suggest an explanation for the effect of temperature on solubility. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) Suggest why chlorine is not often added to swimming pools directly. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
12EP04

– 5 –
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
Turn over
(Question 1 continued)
(e) HOCl ionizes to form the hypochlorite ion, OCl
−
, which is a less effective disinfectant
than the undissociated acid.
The graph shows the concentrations of HOCl (aq) and OCl
−
(aq) at different pH values
at 25
C.
100
90
80
70
60
50
40
30
20
10
0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Fraction / %
pH
HOCl
OCl
−
Deduce the pH range where the water is most effectively sterilized. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(f) Ammonia released from sweat and urine reacts with HOCl to form a range of
compounds including chloramines.
(i) Deduce an equation for the formation of dichloramine, NHCl
2
(aq), from ammonia
and HOCl (aq). [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
12EP05

– 6 –
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
(Question 1 continued)
(ii) The graph shows the molar ratio of chloramines formed at different pH values at
25
C. Trichloramine, NCl
3
, causes pool water to smell bad.
NCl
3
NHCl
2
NH
2
Cl
100
75
50
25
0
2
3
4 5
6
7
8
Molar ratio / %
pH
State two conditions needed to prevent the bad smell. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(g) Suggest two reasons why operating a swimming pool at a lower temperature is
favourable for the environment. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12EP06

– 7 –
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
Turn over
2. A student investigates the effect of exposure to the air on the ascorbic acid (vitamin C)
concentration in a specific orange juice. Equal volumes of orange juice are sealed into
identical flasks and placed in a refrigerator for two weeks. The samples in the refrigerator are
exposed to the air by removing the stopper for a different number of hours each day as shown.
0 h 1 h 2 h 8 h 24 h
Stopper
Flask
Juice
(a) Identify two variables that are controlled. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) The concentration of ascorbic acid is determined by titration with a standard iodine
solution. Every few days, 10.00 cm
3
of orange juice is removed from each sample,
diluted to 100.0 cm
3
, and titrated.
(i) Suggest why the juice is diluted before titration. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Identify a possible systematic error with this method regarding the sample that is
exposed for zero hours. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Suggest how an additional flask could be set up to verify whether the systematic
error in (ii) has occurred. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
12EP07

– 8 –
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
(Question 2 continued)
(c) The following data are collected during a titration.
Final burette reading = 16.10 ± 0.05 cm
3
Initial burette reading = 1.10 ± 0.05 cm
3
Calculate the percentage uncertainty of the titre. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) The following graph shows the student’s results.
+
+
+
+
+
+
+
+
+
+
+
+
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
0.0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
4.5
5.0
5.5
6.0
0 h
1 h
2h
8 h
24 h
Time / d
Daily
exposure
to air
[ascorbic acid] / × 10
–
3
mol
dm
–
3
(i) Calculate the average rate of decrease in ascorbic acid concentration for the 24 h
sample over the period of 14 days, including units. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
12EP08

– 9 –
SPEC/4/CHEMI/SP1/ENG/TZ0/XX
0000 – 6105
(Question 2 continued)
(ii) The student’s hypothesis is: “A lower ascorbic acid concentration will be found in
juice exposed to the air for longer, due to oxidation of ascorbic acid by oxygen.”
Discuss whether or not the data support the hypothesis. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) State the implications of the results of the experiment for avoiding loss of
vitamin C in the storage of orange juice. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(e) Suggest an extension to the investigation that would generate further recommendations
for the storage of orange juice. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12EP09

Disclaimer:
Content used in IB assessments is taken from authentic, third-party sources. The views expressed within them belong to their
individual authors and/or publishers and do not necessarily reect the views of the IB.
References:
1(c) National Center for Biotechnology Information, 2020. PubChem Compound Summary for CID 24526, Chlorine
[online] Available at: <https://pubchem.ncbi.nlm.nih.gov/compound/Chlorine> [Accessed 23 September 2020].
1(e) Norlex, 2020. Chlorine for water disinfection. [online] Available at: <https://norlexpoolspa.com/guidance/about-the-
right-water-balance/safe> [Accessed 23 September 2020]
1(f)(ii) ResearchGate, 2015. Distributions of chloramines as a function of pH. [image online] Available at: <https://
www.researchgate.net/gure/Distribution-of-chloramines-as-a-function-of-pH_g8_273449675> [Accessed
23 September 2020]
12EP10

Please do not write on this page.
Answers written on this page
will not be marked.
12EP11
Please do not write on this page.
Answers written on this page
will not be marked.
12EP12

6 pages
Markscheme
Specimen paper
Chemistry
Standard level
Paper 1 – Section B
– 2 –
This markscheme is the property of the International Baccalaureate
and must not be reproduced or distributed to any other person
without the authorization of the IB Global Centre, Cardiff.

– 3 –
Question
Answers
Notes
Total
1.
(a)
Reactant: Cl
2
0
Products: HOCl +1
HCl -1
Award [2] for three correct.
Award [1] for any two correct.
2
1.
(b)
equilibrium shifts to the right/product AND HCl/HOCl/H
+
removed/neutralized
«by NaOH»
Accept any suitable equation to
illustrate the neutralization reaction.
1
1.
(c)
i
Any one of:
pressure not given
use of different units
OR
different ways of measuring
different precisions/significant figures
OR
uncertainties not given
1 max
1.
(c)
ii
0.177 «dm
3
» × 2.86 «g dm
-3
» / 0.506 «g»
OR
13
0.506«g»
70.9«g mol » 0.100«dm »
−
×
0.0714 «mol dm
-3
»
Award [2] for correct final answer.
Accept use of PV = nRT to calculate the
solubility. Using P = 100 kPa gives a
solubility of 0.0703 «mol dm
-3
».
2
– 4 –
1.
(c)
iii
«as temperature increases solubility decreases»
dissolution is exothermic «hence equilibrium shifts to reactants side at higher
temperatures»
OR
thermal energy overcomes intermolecular forces between chlorine and water
OR
negative entropy change «of dissolution» becomes more dominant at higher
temperatures
Accept “kinetic energy increases with
temperature «so more gas molecules
escape»”.
1
1.
(d)
Any one of:
toxic
gas
difficult to handle/store
1 max
1.
(e)
0 - 6
Accept any number or range below
pH 6.5.
1
1.
(f)
i
NH
3
(aq) + 2HOCl(aq) NHCl
2
(aq) + 2H
2
O(l)
1
1.
(f)
ii
pH > 5/high
low concentration of Cl
2
/HOCl/NH
3
Do not accept general statements such as
“less urination in the pool”.
2
1.
(g)
Any two of
lower water evaporation
reduce energy consumption/less energy needed to heat the water
higher solubility of chlorine «so less chlorine lost»
Accept “less chlorine needed AND
fewer bacteria”.
2 max

– 5 –
Question
Answers
Notes
Total
2.
(a)
Any two for [1 max]
type of orange juice
temperature
light intensity
«initial» surface area
OR
«initial» volume AND flask
1
2.
(b)
i
Any one of:
to perform multiple titrations
too concentrated «so using too much iodine solution»
end-point colour easier to see
1
2.
(b)
ii
flask has to be opened to withdraw samples «so not 0 h»
OR
air was present in the flask at the start «so the ascorbic acid was
exposed to air»
1
2.
(b)
iii
titrate only once after two weeks
OR
fill flask with nitrogen/argon/inert gas
OR
withdraw samples with syringe «without opening flask»
1
2.
(c)
3
3
0.1 cm
« 100 » 0.7 «%»
15.0 cm
×=
1
2.
(d)
i
33 33
4
(5.3 10 mol dm 1.1 10 mol dm )
« »3.0 10
14d
−− −−
−
× −×
= ×
mol dm
-3
d
-1
Accept values in the range 2.9 × 10
-4
-
3.1 × 10
-4
.
Accept values converted to other units,
such as 3.4 × 10
-9
- 3.6 × 10
-9
mol dm
-3
s
-1
or 0.29 - 0.31 mmol dm
-3
d
-1
.
2

– 6 –
2.
(d)
ii
«support» longer daily exposure leads to lower concentration of ascorbic acid
OR
0 h decreases the least
«doesn’t support» no direct evidence of oxidation by oxygen
2
2.
(d)
iii
should be stored in sealed container
OR
should be consumed in a few days after opening
1
2.
(e)
Any one of:
effect of temperature/light AND would show the value of refrigeration/darkness
effect of preservative
compare types of orange juice «e.g. fresh, from concentrate, etc.»
1

Candidate session number
© International Baccalaureate Organization 2023
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
16 pages
Specimen paper
1 hour 30 minutes
Chemistry
Standard level
Paper 2
Instructions to candidates
y Write your session number in the boxes above.
y Do not open this examination paper until instructed to do so.
y Answer all questions.
y Answers must be written within the answer boxes provided.
y A calculator is required for this paper.
y A clean copy of the chemistry data booklet is required for this paper.
y The maximum mark for this examination paper is [50 marks].
16EP01

Please do not write on this page.
Answers written on this page
will not be marked.
– 2 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
16EP02

– 3 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
Turn over
Answer all questions. Answers must be written within the answer boxes provided.
1. A monoprotic acid, HX, is found to have the following composition by mass:
C = 39.99 % H = 6.73 % O = 53.28 %
(a) Determine the empirical formula of the compound HX. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) 25.00 cm
3
of a solution, containing 1.51 g of HX is titrated with a 0.750 mol dm
-
3
solution of NaOH (aq). The HX (aq) solution is exactly neutralized by 22.30 cm
3
of the
NaOH (aq) solution. Determine the molar mass (M) of HX. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) State the molecular formula of HX. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) HX reacts with aqueous sodium hydroxide according to the equation:
HX (aq) +NaOH(aq)→NaX(aq)+ H
2
O (l)
Identify a functional group present in HX. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
16EP03

– 4 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
2. Scientific models are used to explain the structure of matter.
(a) An α-particle is a helium-4 nucleus. In an experiment, α-particles are accelerated
towards a thin sheet of gold and their resulting paths are detected, giving evidence of
the positive charge of the nucleus.
Thin sheet of gold
Path
II
α
-particles
Accelerated
Path
I
Angle of detection θ increase from 0° to 180°
θ
The number of α-particles detected at different angles of deflection θ are shown.
180° 180°90° 0° 90°
Path
II Path II
Path I
θ
Number of
α-
particles detected
Key:
(This question continues on the following page)
16EP04

– 5 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
Turn over
(Question 2 continued)
(i) State the nuclear charges of gold and helium. [1]
Gold: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Helium: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Explain why some α-particles follow path II, rebounding from the gold sheet. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Most of the α-particles follow path I and pass straight through
undeflected (θ = 0°). Suggest a conclusion that can be made about the structure
of the atom based on this evidence. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
16EP05

– 6 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
(Question 2 continued)
(b) Helium was first identified by analysing spectra of solar radiation.
(i) Outline the appearance of the emission spectrum of helium. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Emission spectra of one-electron systems can be explained using a model with
the electron attracted to the nucleus by an electrostatic force.
This model predicts that the electron occupies discrete energy levels. Some energy
levels for the He
+
ion are shown.
0
n
∞
n 3
n 2
n 1
328
582
1310
5250
Energy / kJ mol
1
(ii) Explain how the frequencies observed in emission spectra support the idea of the
electron occupying discrete energy levels. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
16EP06

– 7 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
Turn over
(Question 2 continued)
The ionization energy of the He
+
ion is 5250 kJ mol
-
1
and the ionisation energy of
hydrogen is 1312 kJ mol
-
1
.
(iii) Suggest two reasons why the ionization energy of the hydrogen atom is
significantly smaller than the ionization energy of the He
+
ion. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iv) Suggest why the model outlined in (b)(ii) can predict the emission spectrum of
He
+
but not He. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) Outline why models of the atom have evolved over time. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
16EP07

– 8 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
3. In a simulation, equal masses of potassium and lithium are added to water and the time
taken for the metals to fully react is recorded. Five different increasing masses of each metal
are used, and the reaction is timed.
(a) Sketch the graphs on the axes to show the expected results of this experiment. [2]
Mass of potassium / g
Time for metal to react completely
Mass of lithium / g
Time for metal to react completely
(b) Suggest a reason why comparing the time for complete reaction of equal masses is not
a valid measure of reactivity. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(c) Lithium carbide, Li
2
C
2
, is one of many compounds of lithium and carbon. Determine the
percentage covalent character and bonding type in this compound by using sections 9
and 17 of the data booklet. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
16EP08

– 9 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
Turn over
(Question 3 continued)
(d) Draw the Lewis formula of the anion in the salt Li
2
C
2
. [2]
16EP09

Please do not write on this page.
Answers written on this page
will not be marked.
– 10 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
16EP10
– 11 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
Turn over
4. Heptadecane, C
17
H
36
, can be extracted from crude oil or cactus plants.
(a) Write an equation for the complete combustion of C
17
H
36
. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) The enthalpy of combustion of C
17
H
36
is -11 350 kJ mol
-
1
.
(i) Calculate the maximum energy produced when 2.00 g of C
17
H
36
is combusted. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Determine the maximum temperature change when 500.0 cm
3
of water is heated
by a 2.00 g sample of C
17
H
36
. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Outline two assumptions made in the calculation in (b)(ii). [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
16EP11

– 12 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
(Question 4 continued)
(c) Explain why biofuels contribute less to climate change than fossil fuels. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(d) Heptadecane can be broken down into smaller molecules. Consider the reaction:
C
17
H
36
(g)→2C
2
H
4
(g) + C
13
H
28
(g)
Determine the standard enthalpy change, ΔH
Ö
, for the reaction stated, using section 12
of the data booklet. [3]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(e) Ethene can be converted to ethanol in one reaction. State the equation for this reaction. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
16EP12

– 13 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
Turn over
(Question 4 continued)
(f) Ethanol reacts with oleic acid to produce ethyl oleate.
C
17
H
33
COOH (l) + CH
3
CH
2
OH (l) C
17
H
33
COOCH
2
CH
3
(l) + X (l)
(i) Identify the side product X (l). [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Calculate the atom economy of the reaction. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Discuss why the atom economy of a reaction is an important consideration when
evaluating the impact of a reaction in an industrial process. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
16EP13

– 14 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
5. Halogens are important reactants in the laboratory and in the environment.
(a) (i) Write an equation for the homolytic fission of chlorine under UV light, showing the
movement of electrons. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Under different conditions, chlorine molecules can break down by
heterolytic fission. Write an equation showing the movement of electrons. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(iii) Identify, giving a reason, which one of the three species produced in (a)(i) and
(a)(ii) is an electrophile. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
16EP14

– 15 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
Turn over
(Question 5 continued)
(b) The graph shows the boiling points of the first five straight-chain primary alcohols
and fluoroalkanes.
Relative formula mass
Primary alcohols
Primary fluoroalkanes
Boiling point / °C
150
50
0
50
100
150
20 30 40 50 60 70 80 90 10010
100
Key:
(i) Outline why the alcohols have higher boiling points than fluoroalkanes of similar
relative formula mass. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) Explain the general trend in the boiling points shown for the alcohols. [2]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(This question continues on the following page)
16EP15

– 16 –
SPEC/4/CHEMI/SP2/ENG/TZ0/XX
0000 – 6106
(Question 5 continued)
(c) Hydrochloric acid is an important chemical reactant and industrial chemical.
A pH probe is placed in a small volume of 0.10 mol dm
-
3
solution of hydrochloric acid.
The pH is recorded while a steady stream of distilled water is added to the acid at
constant temperature.
(i) On the axes, sketch the graph of pH against volume of water added. [3]
Volume of H
2
O added
pH
0
4
6
8
10
12
14
2
(ii) The experiment is repeated using 0.010 mol dm
-
3
NaOH (aq) at the same
temperature. State the initial pH of the sodium hydroxide solution. [1]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
16EP16

10 pages
Markscheme
Specimen paper
Chemistry
Standard level
Paper 2
– 2 –
This markscheme is confidential and for the exclusive use of
examiners in this examination session.
It is the property of the International Baccalaureate and must not
be reproduced or distributed to any other person without the
authorization of the IB Global Centre, Cardiff.

– 3 –
General Marking Instructions
Assistant Examiners (AEs) will be contacted by their team leader (TL) through RM™ Assessor, by e-mail or telephone—if through RM™ Assessor or
by email, please reply to confirm that you have downloaded the markscheme from IBIS. The purpose of this initial contact is to allow AEs to raise
any queries they have regarding the markscheme and its interpretation. AEs should contact their team leader through RM™ Assessor or by e-mail at
any time if they have any problems/queries regarding marking. For any queries regarding the use of RM™ Assessor, please contact

– 4 –
Question
Answers
Notes
Total
1. (a)
n
C
= «
1
39.99g
12.01gmol
−
=
» 3.33 «mol»
n
H
= «
1
6.73g
1.01gmol
−
=
» 6.66 «mol»
n
O
= «
1
53.28g
16.00gmol
−
=
=» 3.33 «mol»
CH
2
O
2
1. (b)
n
HX
(= nNaOH =) 0.750 «mol dm
-3
» × 0.02230 «dm
3
» / 0.0167 «mol»
M
HX
= (
1.51 g
0.0167mol
=
) 90.4 g mol
-1
Accept 90.3 «g mol
–1
». 2
1. (c) C
3
H
6
O
3
Accept consistent feasible structural
formula.
1
1.
(d)
carboxyl/COOH
Do not accept “carbonyl/C=O”.
1

– 5 –
Question
Answers
Notes
Total
2.
(a)
(i)
Gold: +79 AND Helium: +2
1
2.
(a)
(ii)
repelled by «hitting/close contact with» gold nucleus
1
2. (a) (iii)
atom is mainly empty space/vacuum
OR
nucleus is very small «compared to the size of the atom»
1
2.
(b)
(i)
discrete/series of lines of different frequency/wavelength
1
2. (b) (ii)
energy of photon relates to a frequency «in the spectrum»
energy of photon depends on difference in energy levels
2
2. (b) (iii)
H half/lower nuclear charge/number of protons
H larger/double radius
2
2.
(b)
(iv)
electron-electron interactions «need to be taken in account»
1
2. (c)
Any one of:
new evidence
new technology
developments in related models
models incomplete/failed to account for all observations
1

– 6 –
Question
Answers
Notes
Total
3. (a)
separate curves/lines for Li and K sketched AND both increasing
steeper gradient for Li
OR
curve/line for Li higher
2
3.
(b)
equal masses «of different substances» do not contain equal amounts/moles
1
3. (c)
«Avg electronegativity = 1.8
Δ electronegativity = 1.6»
% covalent character = 45-55
ionic
2
3. (d)
[:C≡C:]
2-
2- charge
:C≡C:
2

– 7 –
Question
Answers
Notes
Total
4.
(a)
C
17
H
36
(l) + 26O
2
(g) → 17CO
2
(g) + 18H
2
O(l)
1
4. (b) (i)
nC
17
H
36
= «
− −−
= =
× +×
1 11
2.00 g 2.00 g
[(17 12.01g mol ) (36 1.01g mol )] 240.53 g mol
»
0.008315/0.00831 «mol»
« energy = 11350 kJ mol
-1
× 0.008315 mol = » 94.4 «kJ»
2
4. (b) (ii)
94 400 = 500.0 × 4.18 × ∆T
∆T = 45.2«K»
2
4. (b) (iii)
Any two:
water does not evaporate
heat is not lost to the surroundings
OR
all heat is transferred to the water
density of water is 1 g cm
-3
water is pure
complete combustion
2 max

– 8 –
Question
Answers
Notes
Total
4. (c)
CO
2
consumed while plant is growing «and later released when biofuel is
combusted»
OR
photosynthesis uses up CO
2
«later released when biofuel is combusted»
1
4. (d)
bonds broken: 4(C ̶̶ C ) / 4 × 346
bonds formed: 2(C=C) / 2 × 614
ΔH
ϴ
= «4 × 346 – 2 × 614 / 1384 – 1228 =»
«+»156 «kJ»
Award [3] for correct final answer. 3
4.
(e)
C
2
H
4
(g) + H
2
O(g) → C
2
H
5
OH(g)
1
4.
(f)
(i)
H
2
O / water
1
4. (f) (ii)
«20(12.01) + 38(1.01) + 2(16.00) = 310.58
×
=
+
100 310.58
(310.58 18.02)
»
94.5 %
1
4. (f) (iii)
Any two of:
sustainable development
more economical/efficient
better use of natural resources
reduces waste
2 max

– 9 –
Question
Answers
Notes
Total
5. (a) (i)
→ 2 Cl•
2 Cl•
single-barbed/fish-hooks
Accept chlorine atoms. 2
5. (a) (ii)
→
full/double-barbed arrow AND charges on both ions are required for mark.
1
5.
(a)
(iii)
Cl
+
AND can accept a pair of electrons to form a new bond
1

– 10 –
Question
Answers
Notes
Total
5.
(b)
(i)
hydrogen bonding stronger than dipole-dipole
1
5. (b) (ii)
«increase because» stronger London/dispersion forces
more electrons
OR
surface contact
2
5. (c) (i)
start at pH = 1
curve with decreasing gradient
must finish below pH = 7
3
5.
(c)
(ii)
12
1
