
TECHNICAL NOTE 73337
Global round robin test of thiopental EP method
performance on identical HPLC systems
Authors: Sylvia Grosse, Katherine Lovejoy,
Frank Steiner
Thermo Fisher Scientific, Germering, Germany
Keywords: Reproducibility, precision,
European Pharmacopoeia, system suitability
test, UV-Vis, thiopental, variability, small
molecule pharmaceutical
Demonstrated benefits
The presented results show that Thermo Scientific
™
Vanquish
™
Core HPLC systems have excellent system-to-
system reproducibility when the sample preparation, eluent
preparation, and LC column are identical. Users can rely
on the fact that the performance of multiple Vanquish Core
HPLC systems will be highly predictable and robust for
routine methods in quality control labs when other variables
are controlled.
Goals
• Evaluate system-to-system variability, while controlling for
dierent operators, column lots, and solvent grades.
• Present intra- and inter-laboratory precision data for
retention time, peak areas, and relative quantification.
Introduction
High system-to-system reproducibility is critical for high
performance liquid chromatography (HPLC) systems used
for routine analysis in labs where many systems stand
side-by-side, such as in quality control and batch testing
release laboratories. High reproducibility among systems
is also needed for method transfer between labs, such
as for transfer of methods from systems in a research
and development lab and to identical systems in a quality
control lab.
The Vanquish Core HPLC systems are designed for such
routine and universal use. Multiple systems must produce
identical results. In this technical note, we present the
results of a global round robin test designed to evaluate
the system-to-system reproducibility. Multiple HPLC
instruments of the same model were used to analyze
thiopental and its impurities as described by the related
substances method in the current monograph published
by the European Pharmacopoeia (EP).
1
For that purpose,
eight labs and seven operators in four countries on three
continents were equipped with identical HPLC instruments
but dierent pumping technologies and UV detector types
and were asked to perform the exact same analysis.

2
EP certified reference standards and new columns from
dierent batches were used. We report inter- and intra-
laboratory precision data for retention times, peak areas,
relative quantification, and system-to-system variability.
General trends in the eect of eluent preparation, sample
preparation, and column batch on variability were also
explored. The system-to-system reproducibility of the
Vanquish Core HPLC systems was found to be excellent,
especially when eluent, sample, and column variables were
controlled.
Experimental
Chemicals (Germering laboratory)
• Deionized water, 18.2 MΩ·cm resistivity or higher
• Fisher Scientific Acetonitrile, Optima
™
LC/MS grade
(P/N A955-212)
• Fisher Chemical HPLC electrochemical grade ortho-
phosphoric acid 85% (P/N O/0515/PB08)
• EP Certified Reference Standard Thiopental for System
Suitability CRS,
2
containing impurities A, B, C, and D
(P/N Catalogue code Y0001478)
Equipment (Germering laboratory)
• Vials (amber, 2 mL), Fisher Scientific (P/N 03-391-6)
• Cap with Septum (Silicone/PTFE), Fisher Scientific
(P/N 13-622-292)
Instrumentation
• Thermo Scientific Vanquish Core Quaternary and Binary
HPLC systems were used for the analyses, equipped
with:
– System Base Vanquish Core (P/N VC-S01-A)
– Quaternary Pump C (P/N VC-P20-A)
or
– Binary Pump C (P/N VC-P10-A)
– Split Sampler CT (P/N VC-A12-A)
– Column Compartment C (P/N VC-C10-A-03)
– Diode Array Detector CG with standard flow cell, 13 µL
(P/N VC-D11-A with P/N 6083.0510)
or
– Variable Wavelength Detector C with standard flow cell,
11 µL (P/N VC-D40-A with P/N 6077.0250)
Sample preparation
The system suitability standard was prepared as
1 mg/mL thiopental for system suitability CRS, containing
the impurities A, B, C, and D, in mobile phase. A 2 mg
portion of the EP reference standard for system suitability
was weighed in a 2 mL volumetric flask. The flask was then
filled to 2 mL with mobile phase. The standard dissolved
upon vortexing for about 1 minute.
Mobile phase preparation
The mobile phase was prepared by adding 1 g phosphoric
acid (85%) to 900 mL of water in a 1000 mL volumetric
flask and filling to 1000 mL with water. A 350 mL portion of
acetonitrile was added to 650 mL of the phosphoric acid
solution in an eluent bottle, mixed by inverting the bottle
several times until a clear solution became visible, and
degassed by placement for five minutes in an ultrasonic
bath.
Parameter Value
Column
Thermo Scientific
™
Hypersil GOLD
™
,
150 × 4.6 mm, 5 µm
(P/N 25005-154630)
Mobile phase
65:35 1 g/L phosphoric acid
(85%) in water:ACN (v:v)
(isocratic, channel A)
Run time 20 min
Flow rate 1 mL/min
Mixer volume 350 µL + 50 µL
Column temperature
25 °C with passive pre-heater
(forced air with fan speed 5)
Autosampler
temperature
4 °C
UV wavelength 225 nm
UV data collection rate 10 Hz
UV response time 0.5 s
Injection volume 10 µL
Table 1. Chromatographic conditions
Chromatography Data System
The Thermo Scientific
™
Chromeleon
™
Chromatography
Data System (CDS), version 7.3 was used for data
acquisition and analysis.

3
Results and discussion
Results from ten repeated runs of the EP compendial
method for thiopental
1
on each of the eight systems with
dierent columns, eluent brands, eluent grades, sample
preparations, operators, and locations around the world
were compared. In addition, results from three systems
were compared when all these variables were controlled.
Systems are equal when sample, eluent, and column
are identical
Three systems were compared under highly controlled
conditions. The same sample, eluent bottle, and column
were moved from system to system. Ten runs were
performed on each system. The results showed that
when the sample preparation, eluent preparation, column,
operator, and site are all identical, the systems tend to
produce equal retention times, peak areas, and peak
resolution.
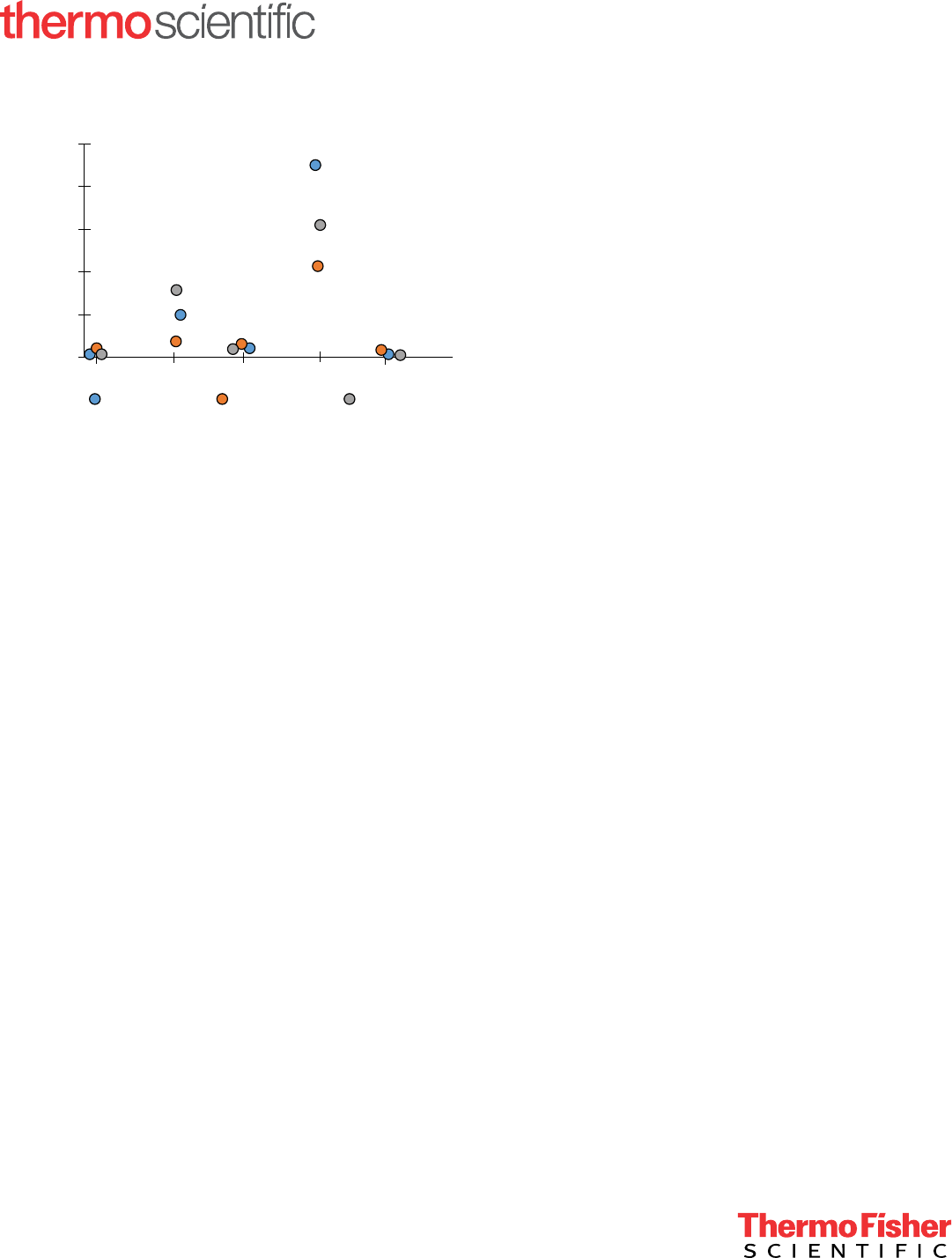
The retention times for thiopental on three systems under
identical conditions are shown in Figure 1. The relative
standard deviation (RSD) for the retention time of thiopental
is 0.4% for the three systems. Because of the controlled
conditions and the high intrinsic system-to-system
reproducibility of the Vanquish Core HPLC system, this
value is nearly an order of magnitude better than the 3.5%
RSD obtained for the eight systems in the global test.
Figure 1. Retention times for thiopental are nearly identical on three
dierent systems when the sample preparation, eluent preparation,
column, operator, and site are identical.
controlled conditions, resulting in peak area RSDs of less
than 2.3% for four of the five peaks. The special case of
impurity D is discussed in the next section. During the
multi-site test, although the peak area measurements
were very precise in each lab, the peak areas diered
widely between labs. As presented in the next section, this
dierence was attributed to dierent sample preparations.
The superb system-to-system reproducibility of the
Vanquish Core HPLC system provides for nearly identical
peak areas when all other conditions are controlled.
Germering 1
Germering 2
Impurity C
Thiopental
Absorbance [mAU]
-10
200
400
650
91
011
Time [min]
Germering 3
2.3%
1.6%
1.7%
1.4%
34%
78%
8%
17%
7%
0%
20%
40%
60%
80%
100%
Peak Area RSD, controlled case (n=3
)
Impurity
A
Thiopenta
l
Impurity
D
Impurity
C
Impurity
B
Peak Area RSD
Peak Area RSD, global (n=8)
Figure 2. Peak area RSDs are much lower for the controlled case
(n = 3) when the sample preparation, eluent preparation, column,
operator, and site are identical, than for the global round robin test
(n = 8) where none of these variables was controlled.
Another measure of system-to-system reproducibility is
performance on the method’s system suitability test. The
system suitability test for thiopental states that a resolution
of at least 1.5 must be obtained for both the impurity C and
thiopental peak pair and the impurity A and impurity B peak
pair. This condition was easily met by all three systems in
the controlled case, as shown in Figure 3, and by all eight
systems in the global test, as discussed in the next section.
0
1
2
3
4
5
6
7
8
9
10
Resolution
R
S
≥1.
5
R
S
(C and Thiopental)
R
S
(A and B)
Germering 3Germering 2Germering 1
Figure 3. The system suitability test for the thiopental method, which
states that the resolution between impurity C and thiopental and
between impurity A and impurity B must be at least 1.5, was easily
met by all three systems in the controlled case. The Hypersil GOLD
column is known for excellent resolution.
The peak areas under the controlled case also show
excellent system-to-system reproducibility. Peak area
reproducibility data for all five peaks are shown in
Figure 2. The peak areas were very similar under the

4
Global test results and eect of sample, eluent,
and column
The global system-to-system test, carried out on
eight dierent systems in four countries, showed
remarkable system-to-system reproducibility. All eight
systems easily passed the system suitability test in the
EP compendial method for thiopental,
1
as shown in
Figure 4. The compendial method also provides
approximate relative retention times (RRTs) for the purpose
of peak identification. The suggested approximate
RRTs and RRTs found in the global test are shown in
Table 2. Because C18 columns vary in hydrophobicity,
polarity, silanol activity, and metal activity, the matches
are not exact, as expected, but were sucient to allow
identification of peaks in the chromatograms. C18 column
properties have been tabulated elsewhere
3
and the RRTs
of the Hypersil GOLD column in this application are not
unexpected based on its characteristics relative to other
C18 columns on the market. All thiopental impurities on all
systems in the global test could be identified based on the
estimated RRTs provided in the compendial method.
Peak area reproducibility as related to sample
preparation
Sample preparation was aected by the non-homogeneous
distribution of solids inside the vials that are sold as the
EP system suitability standard. Although all the sites were
provided with the product as purchased from the EP,
every scoop of the spatula brought up dierent amounts
of each solid. Amounts of each impurity were therefore
dierent in every sample preparation, and these dierences
could not be controlled. The chromatograms in Figure 5
show variation due to in-vial heterogeneity for two dierent
sample preparations on the same system and for the same
sample preparation on three dierent systems.
Impurity
EP-defined
relative retention
(RT
impurity
/RT
thiopental
)
Found relative retention,
average, n=8
(min, max)
A about 0.3 0.53 (0.51, 0.54)
B about 0.4 0.70 (0.62, 0.73)
C about 0.9 0.93 (0.92, 0.93)
D about 1.3 1.27 (1.24, 1.36)
Table 2. Relative retention times found for the global round-robin
test compared to those described by the EP. Relative retentions of
the early eluting impurities A and B were greater than described. Those
of the late-eluting impurities C and D matched the EP description.
Dierences are attributed to the characteristics of the packing of the
Hypersil GOLD column. Because C18 columns vary in properties such as
hydrophobicity, polarity, silanol activity, and metal activity between brand
and manufacturer, reference tables of C18 column characteristics are
available.
3
Figure 4. All eight systems passed the system suitability test,
which required a resolution of at least 1.5 between impurity C and
thiopental.
5 6Time [min]
-5
110
Impurity A
5 6Time [min]
-5
110
Germering 1
Germering 1, dierent sample
Germering 1, controlled case
Germering 2, controlled case
Germering 3, controlled case
Impurity A
Absorbance [mAU]
Absorbance [mAU]
Figure 5. Example of changes in peak area for dierent sample
preparations. The peak for impurity A is shown. On the left side, the same
system is shown with two dierent sample preps. On the right side, three
dierent systems are shown with the same sample prep. The system-to-
system dierence is much smaller than the dierence between two sample
preparations.
0
1
2
3
4
5
6
7
8
9
10
Resolution
Dresden, GER
Ludwigshafen, GER
Shanghai, CN
Bend, USA
Nottingham, UK
Birmingham, UK
Germering 1, GER
Germering 2, GER
R
S
(C and Thiopental)
R
S
(A and B)
R
S
≥1.5

5
Table 3. Peak area RSD for ten injections and average S/N for each
analyte, reported as the minimum and maximum values provided by
the eight global test sites
The sample inhomogeneity had the greatest eect on
the determination of impurity B levels. Impurity A levels
also showed inhomogeneity. The signal-to-noise ratios
(S/N) given in Table 3 show the dierences in sample
preparations. Signal-to-noise was determined using a fixed
one-minute region late in the chromatogram in which no
peaks were present.
Peak name
Peak area RSD
(min and max
of eight sites)
S/N
(min and max
of 8 sites)
Impurity A 0.06%–0.23% 16 54 –11159
Impurity B 0.09%–4.4% 16–724
Impurity C 0.08%–0.22% 757–2033
Impurity D 0.75%–2.0% 32–58
Thiopental 0.03%–0.08% 23427–62738
0.00%
0.05%
0.10%
0.15%
0.20%
0.25%
Impurity C Thiopental
Dresden, GER
Ludwigshafen, GER
Shanghai, CN
Bend, USA
Nottingham, UK
Birmingham, UK
Germering 1, GER
Germering 2, GER
RSD, Peak Area
Figure 6. Peak area reproducibility for impurity C and thiopental in
the global system-to-system test
Figure 7. For the peak of impurity B, a clear relationship between
peak area precision and peak size relative to the baseline is
observed. Peak area RSD is inversely related to signal-to-noise of a given
peak. Data for smaller peaks with very low S/N ratios show very poor
precision, in other words, high peak area RSDs.
Larger peaks had excellent peak area precision, as
shown in Table 3 and Figure 6 for impurity C, impurity A,
and thiopental. Small peaks had a lower signal-to-noise
ratio, which was related to worse peak area precision.
Specifically, a signal-to-noise ratio of less than 60 was
associated with worse peak area precision (above 1%
RSD). For example, the peak area precision of the small
impurity D peak was consistently worse than those of the
other impurities, with RSDs ranging from 0.75 to 2.0%,
and S/N ratios ranging from 32 to 58. For the impurity B
peak, which was present in widely varying amounts in the
samples, the relationship of S/N and peak area precision
is shown in Figure 7. The dierences in amount of impurity
B only reflect variation in sample preparation and do not
indicate system instability.
Retention time reproducibility as related to eluent
preparation
Every site prepared eluents by adding phosphoric acid
(85%) by weight and adding the water and acetonitrile by
volume. Even within the same lab, as shown in Figure 8,
slight dierences in eluent preparation had more influence
on retention times than column lot or system-to-system
variability. In other words, the retention time of thiopental
diers more with dierent eluent preparations on the same
system than on dierent systems with the same eluent
preparation.
0
100
200
300
400
500
600
700
800
0.0%
1.0%
2.0%
3.0%
4.0%
5.0%
Signal-to-Noise Ratio
RSD, Peak Area
Dresden, GER
Ludwigshafen, GE
R
Shanghai, CN
Bend, USA
Nottingham, UK
Birmingham, UK
Germering 1, GER
Germering 2, GER

6
Retention time is largely independent of column
packing material lot
In an eort to consider the eects of column packing lot,
retention times from the global system-to-system tests
were examined. Figure 9 allows comparison of retention
times for all analytes as a function of column lot. Three
column lots were used in the global test and are identified
as Lot A, B, or C. The retention times vary somewhat,
but do not strongly correlate with column lot dierences.
Because the column-to-column dierence is so minor,
dierences in retention time were attributed to eluent
preparation, as discussed above.
Data on individual system components
Autosampler performance
The peak area precision for the controlled case with three
systems side-by-side in the same lab demonstrates the
excellent performance of the autosampler (Figure 10).
The RSDs from ten injections per system show that this
autosampler easily delivers a 0.05%–0.15% RSD for peak
area precision when peak areas are large and signal-to-
noise is above 1000, as observed for thiopental, impurity A,
and impurity C. Minor variations in peak integration aect
smaller peaks more than larger ones and these variations
impact peak area precision.
5.0
6.0
6.0
9.0
9.0
10.5
12.0
15.0
9.5
11.5
Impurity A
Impurity B
Impurity C
Impurity D
Thiopental
Retention time [min]
5.5
8.0
7.0
10.0
9.5
14.0
13.0
10.5
Lot
A
Lot
A
Lot
A
Lot
A
Lot
A
Lot
B
Lot
C
Lot
C
Lot
C
Lot
C
Lot
C
9
10
11
Time [min]
-10
200
400
650
Impurity C
Thiopental
Germering 1, Eluent Prep A
9 10
11
Time [min]
-10
200
400
650
Germering 1, Eluent Prep B Germering 2, Eluent Prep D
Germering 2, Eluent Prep C
Germering 2, Eluent Prep F
Germering 1, Eluent Prep F
Impurity C
Thiopental
Impurity C
Thiopental
Absorbance [mAU]
-10
200
400
650
91011
Time [min]
Absorbance [mAU]
Absorbance [mAU]
Figure 9. Comparison of retention times for all analytes measured
on columns packed with three dierent solid phase lots. Data from
the global tests are included. The general trend in retention times is
independent of column lot and suggests more of a dependence on eluent
preparation.
Figure 8. Three examples of changes in retention time are shown for five dierent eluent preparations and two dierent
systems. The peaks for thiopental and impurity C are shown. The best retention time reproducibility is found when the eluent
preparations are identical. For example, the retention time of the thiopental peak on system 1 (left pane) and system 2 (middle pane)
shows greater dierences between eluent preps than between systems (right pane). The retention times dier more with dierent
eluent preps than with dierent systems.
For Research Use Only. Not for use in diagnostic procedures. © 2020 Thermo Fisher Scientific Inc. All rights reserved. All trademarks
are the property of Thermo Fisher Scientific and its subsidiaries. This information is presented as an example of the capabilities of Thermo
Fisher Scientific Inc. products. It is not intended to encourage use of these products in any manners that might infringe the intellectual
property rights of others. Specifications, terms and pricing are subject to change. Not all products are available in all locations. Please
consult your local sales representative for details. TN73337-EN 0220S
Find out more at thermofisher.com/vanquishcore
0.0%
0.5%
1.0%
1.5%
2.0%
2.5%
Peak Area RSD
Germering 1 Germering 2 Germering 3
B
D
Thiopental
A C
Pump type
The Vanquish Core HPLC system oers both binary and
quaternary pumps and the results of both pump types
were compared. The same excellent retention time and
peak area precision were found, regardless of pump type.
Relative areas for thiopental were also identical, as was
signal-to-noise ratio for thiopental.
Dierences based on pump type were not expected. The
biggest dierence between the two pump types is the
gradient production, but this method required no gradient
and used a pre-mixed solvent in channel A.
Conclusions
• The Vanquish Core HPLC systems have excellent
system-to-system reproducibility for retention time and
peak area, as shown on eight systems in four countries
on three continents.
• Retention time reproducibility is largely governed by
eluent preparation. Column lot is less important. When
identical eluents are used, the RSD for the retention time
of thiopental and impurities on three dierent systems is
never more than 0.4%.
• Peak area reproducibility depends largely on the sample
preparation, which was inhomogeneous due to peculiar
characteristics of the EP standard product used. When
identical samples are used, the RSD for the peak area
of thiopental and four of five impurities on three dierent
systems is never greater than 2.3%.
• The system suitability test criteria for thiopental are easily
met, no matter where in the world the Vanquish Core
HPLC system operates.
References
1. “Thiopental sodium and sodium carbonate.” European Pharmacopoeia 9.0,
07/2012:0212.
2. European Directorate for the Quality of Medicines & HealthCare; European
Pharmacopoeia (Ph. Eur.); 7, Allée Kastner CS 30026, F-67081 Strasbourg (France).
3. “Comparison Guide to C18 Reversed Phase HPLC Columns.” MacMod Analytical Inc.,
Fourth Edition, June 2008, available at http://www.mac-mod.com/pdf/technical-
report/036-ColumnComparisonGuide.pdf . Accessed November 27, 2019.
Figure 10. The peak area precision for the largest peaks of the
controlled case showcases the performance of the autosampler.
The average of the three systems for RSDs of peak areas from ten
injections per system are shown. The peak area precision of 0.05%
RSD for thiopental, 0.06% for impurity A, and 0.12% for impurity C show
outstanding sampling precision. The average RSDs for the smaller peaks
of impurity B and impurity D were 0.49% and 1.6%. Smaller peaks show
worse peak area precision than larger peaks because of dierences
in peak integration, which was done automatically by the Chromeleon
processing method.
