
Proceedings of Machine Learning Research 182:1–24, 2022 Machine Learning for Healthcare
Contrastive Learning of Medical Visual Representations
from Paired Images and Text
Yuhao Zhang
∗
[email protected]anford.edu
Biomedical Informatics Training Program, Stanford University
Hang Jiang
∗
hjian42@stanford.edu
Symbolic Systems Program, Stanford University
Yasuhide Miura
†
ysmiura@stanford.edu
Computer Science Department, Stanford University
Christopher D. Manning manning@stanford.edu
Computer Science and Linguistics Departments, Stanford University
Curtis P. Langlotz langlotz@stanford.edu
Department of Radiology, Stanford University
Abstract
Learning visual representations of medical images (e.g., X-rays) is core to medical image
understanding but its progress has been held back by the scarcity of human annotations.
Existing work commonly relies on fine-tuning weights transferred from ImageNet pretrain-
ing, which is suboptimal due to drastically different image characteristics, or rule-based
label extraction from the textual report data paired with medical images, which is inaccu-
rate and hard to generalize. Meanwhile, several recent studies show exciting results from
unsupervised contrastive learning from natural images, but we find these methods help
little on medical images because of their high inter-class similarity. We propose ConVIRT,
an alternative unsupervised strategy to learn medical visual representations by exploiting
naturally occurring paired descriptive text. Our new method of pretraining medical image
encoders with the paired text data via a bidirectional contrastive objective between the
two modalities is domain-agnostic, and requires no additional expert input. We test Con-
VIRT by transferring our pretrained weights to 4 medical image classification tasks and
2 zero-shot retrieval tasks, and show that it leads to image representations that consid-
erably outperform strong baselines in most settings. Notably, in all 4 classification tasks,
our method requires only 10% as much labeled training data as an ImageNet initialized
counterpart to achieve better or comparable performance, demonstrating superior data
efficiency.
∗
The first two authors contributed equally. YZ is now affliated with AWS AI Labs, while the work was
done before his current affiliation. HJ is now affliated with Massachusetts Institute of Technology.
†
YM is now affiliated with FUJIFILM Corporation.
© 2022 Y. Zhang, H. Jiang, Y. Miura, C.D. Manning & C.P. Langlotz.

Contrastive Learning of Medical Visual Representations from Paired Images and Text
1. Introduction
Medical image understanding has the potential to transform healthcare and has seen rapid
progress with deep learning (Gulshan et al., 2016; Esteva et al., 2017; De Fauw et al.,
2018; Rajpurkar et al., 2018b). Yet, with expert-level performance achieved only in some
specialties and under some circumstances, medical image understanding remains a difficult
task, with classifications dependent on subtle visual distinctions in overall similar images.
This is further exacerbated by the extreme scarcity of annotated data.
Severe cardiomegaly
is noted in the image
with enlarged…
Radiograph shows
pleural effusion in
the right…
Figure 1: Two example chest X-ray images with different abnormality categories, along
with sentences from their paired textual report and example views indicative of their char-
acteristics.
Existing work has followed two general approaches to obtain annotations for medical
imaging tasks. The first approach has been using high-quality annotations created by med-
ical experts (Abr`amoff et al., 2016; Gulshan et al., 2016; Shih et al., 2019; Wang and Wong,
2020). However, the high cost of this approach has resulted in datasets that are mostly
orders of magnitude smaller than natural image datasets such as ImageNet (Russakovsky
et al., 2015). To remedy this, existing work has relied heavily on transferring model weights
from ImageNet pretraining (Wang et al., 2017; Esteva et al., 2017; Irvin et al., 2019). This
approach is suboptimal because, as shown in Figure 1, medical image understanding often
requires representations of very fine-grained visual features that are drastically different
from those required for identifying objects in natural images. As a result, Raghu et al.
(2019) found that ImageNet pretraining often provides little to no benefit compared to
simple random initialization.
A second popular approach is to use expert-crafted rules to extract labels from the
textual reports accompanying the images. This approach has led to datasets of larger
scale, since the text data paired with medical images are often produced naturally by
medical experts in their routine workflow and abundant in a typical hospital’s IT systems.
Nevertheless, this rule-based label extraction approach has two key limitations: 1) the
rules are often inaccurate and limited to a few categories (Wang et al., 2017), leading to
very inefficient use of the textual report data; 2) these rules are often domain-specific and
sensitive to the style of the text, making cross-domain and cross-institution generalization
difficult (Irvin et al., 2019).
2

Contrastive Learning of Medical Visual Representations from Paired Images and Text
In efforts to make more efficient use of unlabeled image data, several recent studies have
shown promising results from contrastive representation learning from natural images (Chen
et al., 2020a; He et al., 2020; Grill et al., 2020). However, as we will show, applying these
image view–based contrastive methods to medical images provides only marginal benefits
compared to ImageNet pretraining, a result mostly due to the high inter-class similarity of
the medical images as in Figure 1.
In this work, we introduce a new method to improve visual representation learning on
medical images by combining the benefits of both learning from abundant textual data
and unsupervised statistical approaches. We present Contrastive VIsual Representation
Learning from Text (ConVIRT), a framework for learning visual representations by exploit-
ing the naturally occurring pairing of images and textual data. ConVIRT improves visual
representations by maximizing the agreement between true image-text pairs versus random
pairs via a bidirectional contrastive objective between the image and text modalities. We
apply ConVIRT to the pretraining of medical image encoders, and show that it leads to
higher-quality in-domain image representations that capture the subtlety of visual features
required for medical image understanding tasks.
Compared to existing methods, ConVIRT has the advantages of utilizing the paired
text data in a way agnostic to the medical specialty and requiring no additional expert
input. This allows us to evaluate ConVIRT by transferring our pretrained encoder weights
to 4 different medical image classification tasks covering 2 medical specialties. We find that
the resulting models outperform all baseline initialization approaches, including the widely
used ImageNet pretraining and strong baselines that also utilize the paired text data. It
further improves upon popular image-only unsupervised learning methods such as SimCLR
(Chen et al., 2020a) and MoCo v2 (Chen et al., 2020b). Most notably, in all 4 classification
tasks, ConVIRT requires only 10% as much labeled training data as an ImageNet initialized
counterpart to achieve better or comparable performance. We further evaluate ConVIRT
on two new zero-shot retrieval tasks, an image-image and a text-image retrieval task, and
also find it superior to all baselines.
Since its original release in 2020, ConVIRT has directly inspired subsequent studies
such as the CLIP framework (Radford et al., 2021) and the ALIGN model (Jia et al., 2021),
which showed that direct adaptations of ConVIRT-style pretraining at much larger scales
lead to state-of-the-art general visual recognition capabilities. To facilitate future research,
we make our model and the collected retrieval datasets
1
publicly available.
1.1. Generalizable Insights about Machine Learning in the Context of
Healthcare
Healthcare data is usually scarce and costly to annotate compared to data in the general
domain. As a result, machine learning models built with a single modality of healthcare
data often face the generalization challenge due to small sample sizes of training data.
Meanwhile, healthcare data is often naturally paired with multimodal clinical features,
including text descriptions or patient metadata, which can be exploited to reduce the cost
of building reliable machine learning models. Our method, ConVIRT, demonstrates an
application of this idea to learning robust medical image encoders by reusing descriptive
1. https://github.com/yuhaozhang/convirt
3
Contrastive Learning of Medical Visual Representations from Paired Images and Text
text naturally produced by experts via a cross-modality learning framework. We show
that this simple method can greatly benefit downstream predictive tasks with reduced
annotation cost. Since the release of our work, similar image-text pretraining strategies
have been used to improve more downstream healthcare tasks including image regeneration
(Wang et al., 2021), medical visual question answering (Eslami et al., 2021) and clinical
risk prediction (Zang and Wang, 2021), etc. Moreover, a similar idea can be extended to
include other modalities of healthcare data, including multiomics data (Han et al., 2021)
or patient metadata (Vu et al., 2021), for more robust and cost-effective machine learning
applications in the healthcare domain.
2. Related Work
Our work is most relevant to work on medical image classification, which we have discussed
in Section 1, and textual report generation from medical images (Wang et al., 2018; Jing
et al., 2018; Liu et al., 2019; Miura et al., 2021). A dominant approach for initializing medical
image encoders in relevant studies has been using encoder weights pretrained on ImageNet,
despite the drastic difference in image characteristics (Raghu et al., 2019). Instead, we
propose an alternative in-domain pretraining strategy for medical imaging and compare
different pretraining approaches that also use the paired medical reports. Our work is
inspired by the recent line of work on image view-based contrastive learning (H´enaff et al.,
2020; Chen et al., 2020a; He et al., 2020; Grill et al., 2020; Sowrirajan et al., 2021; Azizi et al.,
2021), but fundamentally differs from existing studies by exploiting contrastive learning
using the text modality. As we show in Section 6, the added semantics from the text
data makes contrastive learning more effective in learning high-quality representations of
medical images. To our knowledge, our work represents the first systematic attempt in this
direction.
Another line of work related to ours is visual-linguistic representation learning (Lu et al.,
2019; Tan and Bansal, 2019; Su et al., 2020). Among existing studies, Ilharco et al. (2021)
and Gupta et al. (2020) explored cross-modality contrastive objectives related to ours, but
for the purpose of probing visual-linguistic models and learning phrase grounding, respec-
tively. Our work differs from most work in visual-linguistic pretraining in several crucial
ways: 1) existing work in visual-linguistic learning focused on learning visual representations
from paired text via a binary contrastive prediction task, whereas we contribute by showing
the superior performance of the new cross-modality NCE objectives in improving visual
representations; 2) existing work has primarily relied on object representations extracted
from image segmentation models in their preprocessing steps, making them less applicable
to medical image understanding tasks where anatomical segmentations are extremely hard
to obtain; 3) while existing work has run evaluation primarily on visual-linguistic tasks such
as visual question answering, we instead focus on evaluation with classification and retrieval
tasks which are at the center of medical image understanding research.
Several concurrent papers have studied the problem of learning visual representations
from text data (Sariyildiz et al., 2020; Desai and Johnson, 2021) on general-domain image
problems. Most notably, since the original release of our work, ConVIRT has been applied
at larger scales in several general visual recognition studies, including the CLIP model
(Radford et al., 2021), which uses a simplified version of the ConVIRT approach, and the
4
Contrastive Learning of Medical Visual Representations from Paired Images and Text
ALIGN model by Jia et al. (2021). These successful applications have confirmed that
ConVIRT is a promising strategy for learning visual representations from human-written
descriptive text, and that it has the potential to further advance the state of the art for
visual recognition tasks.
There are also subsequent studies which mainly focused on medical-domain image prob-
lems. To the best of our knowledge, ConVIRT was the first work that leverages text-image
contrastive loss for pretraining medical visual representations and was followed by numer-
ous papers (Heiliger et al., 2022) that apply multimodal contrastive learning to the medical
imaging domain. Wang et al. (2021) demonstrated the feasibility of such a pretraining strat-
egy across mixed data inputs (image-only, text-only, image-text pairs) in three chest X-ray
applications (i.e., classification, retrieval, and image regeneration). M¨uller et al. (2021)
proposed a similar method, LoVT, for localized medical imaging tasks. Huang et al. (2021)
adapted our method and further proposed GloRIA to contrast image sub-regions and words
in the paired report. Liao et al. (2021) trained image and text encoders by encouraging
the resulting representations to exhibit high local mutual information. Eslami et al. (2021)
proposed PubMedCLIP to better adapt CLIP to the Medical Visual Question Answering
(MedVQA) task. Zang and Wang (2021) applied a similar contrastive learning framework
to clinical risk prediction based on longitudinal electronic health records. Han et al. (2021)
extended ConVIRT to use radiomics features and contrastive learning for pneumonia de-
tection, and Vu et al. (2021) selected positive pairs coming from views of possibly different
images through the use of patient metadata.
3. Methods
3.1. Task Definition
We start by defining our representation learning setting. We assume paired input (x
v
, x
u
)
where x
v
represents one or a group of images, and x
u
represents a text sequence which de-
scribes the imaging information in x
v
. Our goal is to learn a parameterized image encoder
function f
v
, which maps an image to a fixed-dimensional vector. We are then interested in
transferring the learned image encoder function f
v
into downstream tasks, such as classifi-
cation or image retrieval. In this work, we model the encoder function f
v
as a convolutional
neural network (CNN).
We note that paired image-text data (x
v
, x
u
) naturally exists for many medical domains.
Medical experts such as radiologists produce textual descriptions of images as part of their
routine workflow, some of which are also made publicly available (Demner-Fushman et al.,
2016; Johnson et al., 2019).
3.2. Contrastive Visual Representation Learning from Text
An overview of our method, ConVIRT, for learning f
v
is shown in Figure 2. At a high level,
our method converts each input image x
v
and text x
u
into d-dimensional vector representa-
tions v and u respectively, following a similar processing pipeline. For each input image x
v
,
our method starts by drawing a random view
˜
x
v
from x
v
with a sampled transformation
function t
v
∼ T , where T represents a family of stochastic image transformation functions
described later. Next, the encoder function f
v
transforms
˜
x
v
into a fixed-dimensional vector
5

Contrastive Learning of Medical Visual Representations from Paired Images and Text
Image
Encoder
g
v
<latexit sha1_base64="GAV4YTVulUgyr5GIfU91UIjKRos=">AAAB6nicbVBNS8NAEJ3Ur1q/qh69LBbBU0lUtN4KXjxWtB/QhrLZbtKlm03Y3RRK6E/w4kERr/4ib/4bN2kQtT4YeLw3w8w8L+ZMadv+tEorq2vrG+XNytb2zu5edf+go6JEEtomEY9kz8OKciZoWzPNaS+WFIcep11vcpP53SmVikXiQc9i6oY4EMxnBGsj3QfD6bBas+t2DrRMnILUoEBrWP0YjCKShFRowrFSfceOtZtiqRnhdF4ZJIrGmExwQPuGChxS5ab5qXN0YpQR8iNpSmiUqz8nUhwqNQs90xliPVZ/vUz8z+sn2m+4KRNxoqkgi0V+wpGOUPY3GjFJieYzQzCRzNyKyBhLTLRJp5KHcJ3h8vvlZdI5qzvn9fO7i1qzUcRRhiM4hlNw4AqacAstaAOBAB7hGV4sbj1Zr9bborVkFTOH8AvW+xdwJI4B</latexit>
g
u
<latexit sha1_base64="6oNZ+7ql+oIxts8Fb7qUG/jNIc4=">AAAB6nicbVBNS8NAEJ3Ur1q/qh69LBbBU0msaL0VvHisaD+gDWWz3aRLN5uwuxFK6E/w4kERr/4ib/4bN2kQtT4YeLw3w8w8L+ZMadv+tEorq2vrG+XNytb2zu5edf+gq6JEEtohEY9k38OKciZoRzPNaT+WFIcepz1vep35vQcqFYvEvZ7F1A1xIJjPCNZGugtGyahas+t2DrRMnILUoEB7VP0YjiOShFRowrFSA8eOtZtiqRnhdF4ZJorGmExxQAeGChxS5ab5qXN0YpQx8iNpSmiUqz8nUhwqNQs90xliPVF/vUz8zxsk2m+6KRNxoqkgi0V+wpGOUPY3GjNJieYzQzCRzNyKyARLTLRJp5KHcJXh4vvlZdI9qzuNeuP2vNZqFnGU4QiO4RQcuIQW3EAbOkAggEd4hheLW0/Wq/W2aC1Zxcwh/IL1/gVuoI4A</latexit>
Heart size is enlarged…
No abnormality seen …
Clear consolidation at…
t
v
<latexit sha1_base64="BJCglQUP4yO10CbmipceSn1wMIg=">AAAB6nicbVDLSgNBEOyNrxhfUY9eBoPgKeyqaLwFvHiMaB6QLGF2MpsMmX0w0xsIIZ/gxYMiXv0ib/6Ns5tF1FjQUFR1093lxVJotO1Pq7Cyura+UdwsbW3v7O6V9w9aOkoU400WyUh1PKq5FCFvokDJO7HiNPAkb3vjm9RvT7jSIgofcBpzN6DDUPiCUTTSPfYn/XLFrtoZyDJxclKBHI1++aM3iFgS8BCZpFp3HTtGd0YVCib5vNRLNI8pG9Mh7xoa0oBrd5adOicnRhkQP1KmQiSZ+nNiRgOtp4FnOgOKI/3XS8X/vG6Cfs2diTBOkIdsschPJMGIpH+TgVCcoZwaQpkS5lbCRlRRhiadUhbCdYrL75eXSeus6pxXz+8uKvVaHkcRjuAYTsGBK6jDLTSgCQyG8AjP8GJJ68l6td4WrQUrnzmEX7DevwCD8o4O</latexit>
t
u
<latexit sha1_base64="Iknv9Lcla4+yIPheV6JLCuRo+9U=">AAAB6nicbVBNS8NAEJ3Ur1q/qh69BIvgqaRWtN4KXjxWtB/QhrLZbtqlm03YnQgl9Cd48aCIV3+RN/+NmzSIWh8MPN6bYWaeFwmu0XE+rcLK6tr6RnGztLW9s7tX3j/o6DBWlLVpKELV84hmgkvWRo6C9SLFSOAJ1vWm16nffWBK81De4yxibkDGkvucEjTSHQ7jYbniVJ0M9jKp5aQCOVrD8sdgFNI4YBKpIFr3a06EbkIUcirYvDSINYsInZIx6xsqScC0m2Snzu0To4xsP1SmJNqZ+nMiIYHWs8AznQHBif7rpeJ/Xj9Gv+EmXEYxMkkXi/xY2Bja6d/2iCtGUcwMIVRxc6tNJ0QRiiadUhbCVYqL75eXSeesWqtX67fnlWYjj6MIR3AMp1CDS2jCDbSgDRTG8AjP8GIJ68l6td4WrQUrnzmEX7DevwCCbo4N</latexit>
x
v
<latexit sha1_base64="yBFfOEP6JAF7+nJV5Bt8G31S8l4=">AAAB83icbVDLSsNAFL2pr1pfVZduBovgqiQqWncFNy4r2Ac0oUymk3boZBJmJsUS+htuXCji1p9x5984SYOo9cDA4Zx7uWeOH3OmtG1/WqWV1bX1jfJmZWt7Z3evun/QUVEiCW2TiEey52NFORO0rZnmtBdLikOf064/ucn87pRKxSJxr2cx9UI8EixgBGsjuW6I9dgP0of5YDqo1uy6nQMtE6cgNSjQGlQ/3GFEkpAKTThWqu/YsfZSLDUjnM4rbqJojMkEj2jfUIFDqrw0zzxHJ0YZoiCS5gmNcvXnRopDpWahbyazjOqvl4n/ef1EBw0vZSJONBVkcShIONIRygpAQyYp0XxmCCaSmayIjLHERJuaKnkJ1xkuv7+8TDpndee8fn53UWs2ijrKcATHcAoOXEETbqEFbSAQwyM8w4uVWE/Wq/W2GC1Zxc4h/IL1/gWoupIy</latexit>
x
u
<latexit sha1_base64="Nb5HKUZQBqKf5+DJLkiWYRSTWCQ=">AAAB83icbVDLSsNAFL2pr1pfVZduBovgqiRWtO4KblxWsA9oQplMJ+3QySTMQyyhv+HGhSJu/Rl3/o1JGkStBwYO59zLPXP8mDOlbfvTKq2srq1vlDcrW9s7u3vV/YOuiowktEMiHsm+jxXlTNCOZprTfiwpDn1Oe/70OvN791QqFok7PYupF+KxYAEjWKeS64ZYT/wgeZgPzbBas+t2DrRMnILUoEB7WP1wRxExIRWacKzUwLFj7SVYakY4nVdco2iMyRSP6SClAodUeUmeeY5OUmWEgkimT2iUqz83EhwqNQv9dDLLqP56mfifNzA6aHoJE7HRVJDFocBwpCOUFYBGTFKi+SwlmEiWZkVkgiUmOq2pkpdwleHi+8vLpHtWdxr1xu15rdUs6ijDERzDKThwCS24gTZ0gEAMj/AML5axnqxX620xWrKKnUP4Bev9C6c2kjE=</latexit>
˜
x
v
<latexit sha1_base64="A+uyhcaKnYXKh//M0GTTaXFQRzQ=">AAAB/XicbVDLSsNAFJ3UV62v+Ni5CRbBVUmsaN0V3LisYB/QhDCZTNqhk0mYmRRrCP6KGxeKuPU/3Pk3TtIgaj0wcDjnXu6Z48WUCGman1plaXllda26XtvY3Nre0Xf3eiJKOMJdFNGIDzwoMCUMdyWRFA9ijmHoUdz3Jle5359iLkjEbuUsxk4IR4wEBEGpJFc/sCWhPk7tEMqxF6R3WeZOXb1uNswCxiKxSlIHJTqu/mH7EUpCzCSiUIihZcbSSSGXBFGc1exE4BiiCRzhoaIMhlg4aZE+M46V4htBxNVj0ijUnxspDIWYhZ6azEOKv14u/ucNExm0nJSwOJGYofmhIKGGjIy8CsMnHCNJZ4pAxInKaqAx5BBJVVitKOEyx/n3lxdJ77RhNRvNm7N6u1XWUQWH4AicAAtcgDa4Bh3QBQjcg0fwDF60B+1Je9Xe5qMVrdzZB7+gvX8Bo6GWGQ==</latexit>
˜
x
u
<latexit sha1_base64="Qz8ijo3u8eXVAMok1C6FMyzVabA=">AAAB/XicbVDLSsNAFJ34rPUVHzs3g0VwVVIrWncFNy4r2Ac0IUwmk3boZBJmJmINwV9x40IRt/6HO//GSRpErQcGDufcyz1zvJhRqSzr01hYXFpeWa2sVdc3Nre2zZ3dnowSgUkXRywSAw9JwignXUUVI4NYEBR6jPS9yWXu92+JkDTiN2oaEydEI04DipHSkmvu24oyn6R2iNTYC9K7LHMT16xZdasAnCeNktRAiY5rfth+hJOQcIUZknLYsGLlpEgoihnJqnYiSYzwBI3IUFOOQiKdtEifwSOt+DCIhH5cwUL9uZGiUMpp6OnJPKT86+Xif94wUUHLSSmPE0U4nh0KEgZVBPMqoE8FwYpNNUFYUJ0V4jESCCtdWLUo4SLH2feX50nvpN5o1pvXp7V2q6yjAg7AITgGDXAO2uAKdEAXYHAPHsEzeDEejCfj1XibjS4Y5c4e+AXj/QuiHZYY</latexit>
v
<latexit sha1_base64="wYpTtEjfuznTbCRwYanHQYo4qzw=">AAAB8XicbVDLSsNAFL2pr1pfVZduBovgqiS2aN0V3LisYB/YhjKZTtqhk0mYmRRK6F+4caGIW//GnX/jJA2i1gMDh3PuZc49XsSZ0rb9aRXW1jc2t4rbpZ3dvf2D8uFRR4WxJLRNQh7KnocV5UzQtmaa014kKQ48Trve9Cb1uzMqFQvFvZ5H1A3wWDCfEayN9DAIsJ54fjJbDMsVu2pnQKvEyUkFcrSG5Y/BKCRxQIUmHCvVd+xIuwmWmhFOF6VBrGiEyRSPad9QgQOq3CRLvEBnRhkhP5TmCY0y9edGggOl5oFnJtOE6q+Xiv95/Vj7DTdhIoo1FWT5kR9zpEOUno9GTFKi+dwQTCQzWRGZYImJNiWVshKuU1x+n7xKOhdVp1at3dUrzUZeRxFO4BTOwYEraMIttKANBAQ8wjO8WMp6sl6tt+Vowcp3juEXrPcvEASRRw==</latexit>
u
<latexit sha1_base64="2fA20Io5yKS6x+nOQ6m7wA9aXkE=">AAAB8XicbVDLSsNAFL2pr1pfVZduBovgqiRWtO4KblxWsA9sQ5lMJ+3QySTMTIQQ+hduXCji1r9x5984SYOo9cDA4Zx7mXOPF3GmtG1/WqWV1bX1jfJmZWt7Z3evun/QVWEsCe2QkIey72FFORO0o5nmtB9JigOP0543u8783gOVioXiTicRdQM8EcxnBGsj3Q8DrKeen8bzUbVm1+0caJk4BalBgfao+jEchyQOqNCEY6UGjh1pN8VSM8LpvDKMFY0wmeEJHRgqcECVm+aJ5+jEKGPkh9I8oVGu/txIcaBUEnhmMkuo/nqZ+J83iLXfdFMmolhTQRYf+TFHOkTZ+WjMJCWaJ4ZgIpnJisgUS0y0KamSl3CV4eL75GXSPas7jXrj9rzWahZ1lOEIjuEUHLiEFtxAGzpAQMAjPMOLpawn69V6W4yWrGLnEH7Bev8CDn+RRg==</latexit>
f
v
<latexit sha1_base64="2nVUs6VWesxTnIyAKqoHyPJX2fQ=">AAAB6nicbVBNS8NAEJ3Ur1q/qh69LBbBU0ls0XorePFY0X5AG8pmu2mXbjZhd1MooT/BiwdFvPqLvPlv3KRB1Ppg4PHeDDPzvIgzpW370yqsrW9sbhW3Szu7e/sH5cOjjgpjSWibhDyUPQ8rypmgbc00p71IUhx4nHa96U3qd2dUKhaKBz2PqBvgsWA+I1gb6d4fzoblil21M6BV4uSkAjlaw/LHYBSSOKBCE46V6jt2pN0ES80Ip4vSIFY0wmSKx7RvqMABVW6SnbpAZ0YZIT+UpoRGmfpzIsGBUvPAM50B1hP110vF/7x+rP2GmzARxZoKslzkxxzpEKV/oxGTlGg+NwQTycytiEywxESbdEpZCNcpLr9fXiWdi6pTq9bu6pVmI4+jCCdwCufgwBU04RZa0AYCY3iEZ3ixuPVkvVpvy9aClc8cwy9Y719uno4A</latexit>
Text
Encoder
f
u
<latexit sha1_base64="/zuhxpaIXDN+q0BXHa6RoGCv3Hs=">AAAB6nicbVBNS8NAEJ3Ur1q/qh69LBbBU0msaL0VvHisaNpCG8pmu2mXbjZhdyOU0J/gxYMiXv1F3vw3btIgan0w8Hhvhpl5fsyZ0rb9aZVWVtfWN8qbla3tnd296v5BR0WJJNQlEY9kz8eKciaoq5nmtBdLikOf064/vc787gOVikXiXs9i6oV4LFjACNZGuguGybBas+t2DrRMnILUoEB7WP0YjCKShFRowrFSfceOtZdiqRnhdF4ZJIrGmEzxmPYNFTikykvzU+foxCgjFETSlNAoV39OpDhUahb6pjPEeqL+epn4n9dPdND0UibiRFNBFouChCMdoexvNGKSEs1nhmAimbkVkQmWmGiTTiUP4SrDxffLy6RzVnca9cbtea3VLOIowxEcwyk4cAktuIE2uEBgDI/wDC8Wt56sV+tt0VqyiplD+AXr/QttGo3/</latexit>
`
(u!v)
<latexit sha1_base64="yxNgwpygkrs8xqoYf7nyNiMqsFQ=">AAACAXicbVDLSsNAFJ34rPUVdSO4GSxC3ZTEitZdwY3LCvYBTSyT6aQdOpmEmUmlhLrxV9y4UMStf+HOv3GSBlHrgQuHc+7l3nu8iFGpLOvTWFhcWl5ZLawV1zc2t7bNnd2WDGOBSROHLBQdD0nCKCdNRRUjnUgQFHiMtL3RZeq3x0RIGvIbNYmIG6ABpz7FSGmpZ+47hLHbpBxDR9DBUCEhwjs4Pp72zJJVsTLAeWLnpARyNHrmh9MPcRwQrjBDUnZtK1JugoSimJFp0YkliRAeoQHpaspRQKSbZB9M4ZFW+tAPhS6uYKb+nEhQIOUk8HRngNRQ/vVS8T+vGyu/5iaUR7EiHM8W+TGDKoRpHLBPBcGKTTRBWFB9K8RDJBBWOrRiFsJFirPvl+dJ66RiVyvV69NSvZbHUQAH4BCUgQ3OQR1cgQZoAgzuwSN4Bi/Gg/FkvBpvs9YFI5/ZA79gvH8BNwKW2A==</latexit>
`
(v!u)
<latexit sha1_base64="yesXrGIjOdpYYTZLLfLlHJLtxOs=">AAACAXicbVDLSsNAFJ34rPUVdSO4GSxC3ZTEitZdwY3LCvYBTSyT6aQdOpmEmUmlhLrxV9y4UMStf+HOv3GSBlHrgQuHc+7l3nu8iFGpLOvTWFhcWl5ZLawV1zc2t7bNnd2WDGOBSROHLBQdD0nCKCdNRRUjnUgQFHiMtL3RZeq3x0RIGvIbNYmIG6ABpz7FSGmpZ+47hLHbpDyGjqCDoUJChHcwPp72zJJVsTLAeWLnpARyNHrmh9MPcRwQrjBDUnZtK1JugoSimJFp0YkliRAeoQHpaspRQKSbZB9M4ZFW+tAPhS6uYKb+nEhQIOUk8HRngNRQ/vVS8T+vGyu/5iaUR7EiHM8W+TGDKoRpHLBPBcGKTTRBWFB9K8RDJBBWOrRiFsJFirPvl+dJ66RiVyvV69NSvZbHUQAH4BCUgQ3OQR1cgQZoAgzuwSN4Bi/Gg/FkvBpvs9YFI5/ZA79gvH8BNxCW2A==</latexit>
<latexit sha1_base64="jVTJ5jwmBW2P8/jiNRcgM4SQ6Hw=">AAAB9HicbVBNS8NAFHypX7V+VT16WSyCp5JIwR4LXjxWsLbQlLLZvrRLN5uwuymU0L/hxYOCePXHePPfuGlz0NaBhWHmPd7sBIng2rjut1Pa2t7Z3SvvVw4Oj45PqqdnTzpOFcMOi0WsegHVKLjEjuFGYC9RSKNAYDeY3uV+d4ZK81g+mnmCg4iOJQ85o8ZKvh9RMwnCbLIYzobVmlt3lyCbxCtIDQq0h9UvfxSzNEJpmKBa9z03MYOMKsOZwEXFTzUmlE3pGPuWShqhHmTLzAtyZZURCWNlnzRkqf7eyGik9TwK7GSeUa97ufif109N2BxkXCapQclWh8JUEBOTvAAy4gqZEXNLKFPcZiVsQhVlxtZUsSV461/eJN2buteoe95Do9ZqFn2U4QIu4Ro8uIUW3EMbOsAggWd4hTcndV6cd+djNVpyip1z+APn8wcSOJIk</latexit>
h
v
<latexit sha1_base64="Gk/nLwtipW+47R3v6rtxOFnsCpc=">AAAB9HicbVDLSgMxFL1TX7W+qi7dBIvgqsyIYJcFNy4rWFvoDCWTZtrQJDPkIZShv+HGhYK49WPc+Tdm2llo64HA4Zx7uScnzjjTxve/vcrG5tb2TnW3trd/cHhUPz551KlVhHZJylPVj7GmnEnaNcxw2s8UxSLmtBdPbwu/90SVZql8MLOMRgKPJUsYwcZJYSiwmcRJPpkP7bDe8Jv+AmidBCVpQInOsP4VjlJiBZWGcKz1IPAzE+VYGUY4nddCq2mGyRSP6cBRiQXVUb7IPEcXThmhJFXuSYMW6u+NHAutZyJ2k0VGveoV4n/ewJqkFeVMZtZQSZaHEsuRSVFRABoxRYnhM0cwUcxlRWSCFSbG1VRzJQSrX14nvatmcN0MgvvrRrtV9lGFMziHSwjgBtpwBx3oAoEMnuEV3jzrvXjv3sdytOKVO6fwB97nDxCzkiM=</latexit>
h
u
Figure 2: Overview of our ConVIRT framework. The blue and green shades represent
the image and text encoding pipelines, respectively. Our method relies on maximizing the
agreement between the true image-text representation pairs with bidirectional losses ℓ
(v→u)
and ℓ
(u→v)
.
h
v
, followed by a non-linear projection g
v
which further transforms h
v
into vector v:
v = g
v
(f
v
(
˜
x
v
)), (1)
where v ∈ R
d
. Similarly, for each text input x
u
, we obtain a span
˜
x
u
from it following a
sampling function t
u
, and then a text representation u with: u = g
u
(f
u
(
˜
x
u
)), where f
u
is
a text encoder, g
u
a projection, and u ∈ R
d
. The projection functions g
v
and g
u
project
representations for both modalities from their encoder space to the same d-dimensional
space for contrastive learning.
At training time, we sample a minibatch of N input pairs (x
v
, x
u
) from training data,
and calculate their representation pairs (v, u). We use (v
i
, u
i
) to denote the i-th pair.
The training objective of ConVIRT involves two loss functions. The first loss function is an
image-to-text contrastive loss for the i-th pair:
ℓ
(v→u)
i
= − log
exp(⟨v
i
, u
i
⟩/τ)
P
N
k=1
exp(⟨v
i
, u
k
⟩/τ)
, (2)
where ⟨v
i
, u
i
⟩ represents the cosine similarity, i.e., ⟨v, u⟩ = v
⊤
u/∥v∥∥u∥; and τ ∈ R
+
represents a temperature parameter. This loss takes the same form as the InfoNCE loss
(Oord et al., 2018), and minimizing it leads to encoders that maximally preserve the mutual
information between the true pairs under the representation functions. Intuitively, it is the
log loss of an N-way classifier that tries to predict (v
i
, u
i
) as the true pair. Note that
unlike previous work which use a contrastive loss between inputs of the same modality
(Chen et al., 2020a; He et al., 2020), our image-to-text contrastive loss is asymmetric for
each input modality. We therefore define a similar text-to-image contrastive loss as:
ℓ
(u→v)
i
= − log
exp(⟨u
i
, v
i
⟩/τ)
P
N
k=1
exp(⟨u
i
, v
k
⟩/τ)
. (3)
6

Contrastive Learning of Medical Visual Representations from Paired Images and Text
Our final training loss is then computed as a weighted combination of the two losses averaged
over all positive image-text pairs in each minibatch:
L =
1
N
N
X
i=1
λℓ
(v→u)
i
+ (1 − λ)ℓ
(u→v)
i
, (4)
where λ ∈ [0, 1] is a scalar weight.
3.3. Realization
We note that our ConVIRT framework defined above is agnostic to the specific choice of
image and text encoders, transformations and projection functions. Following previous work
(Chen et al., 2020a), we model g
v
and g
u
as separate learnable single-hidden-layer neural
networks, i.e., g
v
(·) = W
(2)
σ(W
(1)
(·)) where σ is a ReLU non-linearity, and similarly for
g
u
.
For the image encoder f
v
, we use the ResNet50 architecture (He et al., 2016) for all
experiments, as it is the architecture of choice for much medical imaging work and is shown
to achieve competitive performance. For the text encoder f
u
, we use a BERT encoder
(Devlin et al., 2019) followed by a max-pooling layer over all output vectors. We initialize
our encoder with the ClinicalBERT weights (Alsentzer et al., 2019) pretrained on the MIMIC
clinical notes, which achieved state-of-the-art performance on a suite of clinical NLP tasks.
At training time we allow the encoder to adapt to our contrastive task by freezing the
embeddings and the first 6 transformer layers of this BERT encoder and fine-tuning the
last 6 layers.
For the image transformation family T where t
v
is sampled from, we use sequential
applications of five random transformations: cropping, horizontal flipping, affine transfor-
mation, color jittering and Gaussian blur. Different from recent work on contrastive visual
learning (Chen et al., 2020a,b), we only apply brightness and contrast adjustments in color
jittering, due to the monochrome nature of the medical images. For the text transformation
function t
u
, we apply a simple uniform sampling of a sentence from the input document x
u
(i.e.,
˜
x
u
is a randomly sampled sentence from x
u
for each minibatch). We did not use a more
aggressive transformation mainly because sampling at the sentence level helps preserve the
semantic meaning of the sampled spans.
An alternative method to using the sampled view
˜
x
v
from x
v
as input to the encoder
is to directly use x
v
or to fuse all images for each study in the case of multiple available
x
v
instances (e.g., images from multiple angles). We empirically found in our preliminary
experiments that using sampled view
˜
x
v
leads to better pretraining results. We conjecture
that we can treat the use of
˜
x
v
as a way of data augmentation for the visual modality,
which helped increase the effective amount of unique image-text pairs that the model sees
at pretraining time, leading to better performance.
4. Experiments
We now introduce the paired datasets that we used for contrastive pretraining, the down-
stream tasks and datasets for evaluation, and the baseline methods that we compare against.
7
Contrastive Learning of Medical Visual Representations from Paired Images and Text
4.1. Data for Pretraining
We evaluate ConVIRT by pretraining two separate image encoders using two separate
image-text datasets (see Appendix A for full pretraining details):
• Chest image encoder: We use version 2 of the public MIMIC-CXR database (Johnson
et al., 2019), which is a collection of chest radiograph images paired with their textual
reports, and since its release has become a standard resource for studying multi-modal
modeling of medical images. After preprocessing, this dataset contains a total of about
217k image-text pairs, with each pair containing an average of 1.7 images and 6.0 sen-
tences.
• Bone image encoder: We obtain a collection of musculoskeletal (i.e., bone) image-text
pairs from the Rhode Island Hospital system. Following chest, musculoskeletal images
constitute the second most common type of radiograph images in a typical hospital. This
dataset contains a total of 48k image-text pairs, with each pair containing an average of
2.5 images and 8.0 sentences.
4.2. Evaluation Tasks & Data
We evaluate our pretrained image encoders on three medical imaging tasks: image classifi-
cation, zero-shot image-image retrieval and zero-shot text-image retrieval.
Image Classification. We evaluate our pretrained image encoders on four representative
medical image classification tasks: 1) RSNA Pneumonia Detection (Wang et al., 2017;
Shih et al., 2019), which involves binary classification of a chest radiograph image into ei-
ther a pneumonia or a normal category; 2) CheXpert image classification (Irvin et al.,
2019), which involves multi-label binary classification of a chest image for five individual la-
bels, i.e., atelectasis, cardiomegaly, consolidation, edema and pleural effusion; 3) COVIDx
(Wang and Wong, 2020), which involves multi-class chest image classification into three cat-
egories (COVID19, non-COVID pneumonia or normal); and 4) MURA bony abnormality
detection (Rajpurkar et al., 2018a), which involves binary classification of a musculoskeletal
image into abnormal or normal. We report test accuracy for COVIDx given its balanced
test set, and report the standard area under the receiver operating characteristic curve
(AUC) metric for other tasks.
Following previous work (H´enaff et al., 2020; Chen et al., 2020a; He et al., 2020), for all
tasks, we evaluate each pretrained image encoder under two settings: a linear classifica-
tion setting, where the pretrained CNN weights are frozen and only a linear classification
head is trained for the task; and a fine-tuning setting, where both the CNN weights and
the linear head are fine-tuned. The two settings complement each other for evaluation pur-
poses: while the linear setting directly evaluates the quality of the extracted image features
with the pretrained CNN, the fine-tuning setting more closely resembles how the pretrained
CNN weights are used in practical applications.
To further compare the data efficiency of different pretraining methods, for each set-
ting we evaluate the image encoders with 1%, 10% and all training data, respectively
(except for the COVIDx task where we omit the 1% setting due to the scarcity of training
data). To control the variance in results, for all settings and models, we report average
8
Contrastive Learning of Medical Visual Representations from Paired Images and Text
results over 5 independent training runs. We include further dataset and training details
in Appendix B.
Zero-shot Image-image Retrieval. This evaluation is similar to the conventional content-
based image retrieval setting in which we search for images of a particular category using a
representative query image. For evaluation, a group of query images and a larger collection
of candidate images, each with a categorical label, are given to a pretrained CNN encoder.
We encode each query and candidate image with this encoder, and then for each query,
rank all candidates by their cosine similarities to the query in descending order. Since a
widely-used annotated benchmark for this setting is not available, we create our own dataset
by re-using existing annotations in the CheXpert dataset (Irvin et al., 2019) and additional
expert annotations from a board-certified radiologist. The resulting dataset covers 8 differ-
ent chest abnormality categories, each with 10 expert-annotated query and 200 candidate
images. We include the detailed collection and annotation procedure in Appendix C, and
refer to this dataset as CheXpert 8×200 Retrieval Dataset. We focus our evaluation on
retrieval precision, and evaluate our models with Precision@k metrics where k = 5, 10, 100.
Zero-shot Text-image Retrieval. This setting is similar to the image-image retrieval
setting, but instead of using query images, we retrieve images of a particular category with
textual queries. For this purpose, we ask a radiologist to write 5 diverse and representative
textual descriptions for each of the 8 abnormality categories for the same CheXpert 8x200
candidate images (see Appendix D for details). At test time, for each query we encode its
text with the learned text encoder f
u
and then retrieve from candidate images in a similar
way. This evaluation not only evaluates the quality of the learned image representations,
but also the alignment between the text representations and the image representations. We
again use Precision@k metrics where k = 5, 10, 100.
4.3. Baseline Methods
We compare ConVIRT against the following standard or competitive initialization methods:
• Random Init.: For all tasks we initialize the ResNet50 with its default random initial-
ization.
• ImageNet Init.: We use CNN weights pretrained on ImageNet (Russakovsky et al.,
2015), which remains a dominant initialization approach for medical imaging work (Raghu
et al., 2019).
• Caption-LSTM: We further pretrain the ImageNet-initialized CNN weights with an
image captioning task using the standard CNN-LSTM with attention model (Xu et al.,
2015). We train the model to decode the paired medical report text from the encoded
image representations. Compared to the random or ImageNet initializations, this is an
“in-domain” initialization baseline which uses the paired text data for representation
learning.
• Caption-Transformer: We use a CNN-Transformer-based captioning model (Cornia
et al., 2020) for caption-based pretraining, which recently achieves state-of-the-art results
on the COCO image captioning benchmark (Lin et al., 2014).
9
Contrastive Learning of Medical Visual Representations from Paired Images and Text
• Contrastive-Binary-Loss: This baseline differs from ConVIRT by contrasting the
paired image and text representations with a binary classification head, as is widely
done in visual-linguistic pretraining work (Tan and Bansal, 2019; Su et al., 2020). For
each input pair, we first project encoder outputs h
v
and h
u
into the same dimension with
linear layers, concatenate them, and use a MLP network to predict a binary probability
of whether the input is a real or a “fake” pair, which we train with a binary cross-entropy
loss. During training, for each (x
v
, x
u
) pair in the training set, we construct a “fake”
pair by replacing x
u
with a randomly sampled one from the dataset. We expect that the
binary classification task requires the encoder to learn reasonable representations of the
input images, and therefore is a stronger in-domain initialization baseline.
For fair comparison, for all baselines that require paired image-text data, we use the
same datasets as in our contrastive pretraining. For the captioning-based methods, we
always use the model checkpoints that achieve the best CIDEr score (Vedantam et al.,
2015) on a held-out validation set.
5. Results
5.1. Classification Tasks
Linear Classification. We present all linear classification results for the four tasks in
Table 1(a). We find that compared to random initialization, ImageNet initialization provides
markedly better representations, despite pretrained on a very different domain of images; in-
domain image initialization methods that use paired image-text data further improve over
ImageNet initialization in almost all settings. Among the in-domain initialization methods,
our proposed ConVIRT pretraining achieves the best overall results in all settings. Notably,
we find on three out of the four tasks, with only 1% training data ConVIRT is able to achieve
classification results better than the default ImageNet initialization with 100% training data,
highlighting the high quality of the learned representations from ConVIRT.
Fine-tuning. We show the fine-tuning evaluation results in Table 1(b). Similar to the
linear setting, we find that: 1) ImageNet initialization is again better than random initial-
ization with smaller margins; 2) all in-domain initialization methods are better than the
popular ImageNet initialization in most settings; and 3) our proposed ConVIRT pretraining
again achieves the best overall results in 10 out of the 11 settings, with the exception of
the CheXpert dataset with all training data used, where the result of ConVIRT is similar
to that of the Caption-Transformer result. Most notably, on all datasets, with only 10%
labeled training data ConVIRT achieves classification results that are better or close to the
ImageNet initialization with 100% training data results.
We also notice that our conclusion of using ImageNet versus random initialization is
different from (Raghu et al., 2019): while they showed comparable results from the two
strategies, we find that using ImageNet initialization is still superior than random initial-
ization in most results, justifying its popularity. Upon closer examination, we conjecture
that this is likely due to under-optimization of their models: while our ResNet50 with ran-
dom initialization achieves an average AUC of 85.8 on the CheXpert dataset, their ResNet50
model only achieved 83.5 AUC on the same evaluation set.
10

Contrastive Learning of Medical Visual Representations from Paired Images and Text
(a) Linear classification
RSNA (AUC) CheXpert (AUC) COVIDx (Accu.) MURA (AUC)
Method 1% 10% all 1% 10% all 10% all 1% 10% all
General initialization methods
Random Init. 55.0 67.3 72.3 58.2 63.7 66.2 69.2 73.5 50.9 56.8 62.0
ImageNet Init. 82.8 85.4 86.9 75.7 79.7 81.0 83.7 88.6 63.8 74.1 79.0
In-domain initialization methods
Caption-Transformer 84.8 87.5 89.5 77.2 82.6 83.9 80.0 89.0 66.5 76.3 81.8
Caption-LSTM 89.8 90.8 91.3 85.2 85.3 86.2 84.5 91.7 75.2 81.5 84.1
Contrastive-Binary-Loss 88.9 90.5 90.8 84.5 85.6 85.8 80.5 90.8 76.8 81.7 85.3
ConVIRT (Ours) 90.7 91.7 92.1 85.9 86.8 87.3 85.9 91.7 81.2 85.1 87.6
(b) Fine-tuning
RSNA (AUC) CheXpert (AUC) COVIDx (Accu.) MURA (AUC)
Method 1% 10% all 1% 10% all 10% all 1% 10% all
General initialization methods
Random Init. 71.9 82.2 88.5 70.4 81.1 85.8 75.4 87.7 56.8 61.6 79.1
ImageNet Init. 83.1 87.3 90.8 80.1 84.8 87.6 84.4 90.3 72.1 81.8 87.0
In-domain initialization methods
Caption-Transformer 86.3 89.2 92.1 81.5 86.4 88.2 88.3 92.3 75.2 83.2 87.6
Caption-LSTM 87.2 88.0 91.0 83.5 85.8 87.8 83.8 90.8 78.7 83.3 87.8
Contrastive-Binary-Loss 87.7 89.9 91.2 86.2 86.1 87.7 89.5 90.5 80.6 84.0 88.4
ConVIRT (Ours) 88.8 91.5 92.7 87.0 88.1 88.1 90.3 92.4 81.3 86.5 89.0
Table 1: Results for the medical image classification tasks: (a) linear classification; (b)
fine-tuning setting. All results are averaged over 5 independent models. Best results for
each setting are in boldface. COVIDx 1% setting is omitted due to the scarcity of labels in
COVIDx.
5.2. Retrieval Tasks
We present the zero-shot image-image and text-image retrieval results in Table 2. For the
image-image retrieval setting, we present additional results from fine-tuning our pretrained
model on all CheXpert training data, and use them as “upper bounds” of the results ob-
tained from the use of supervised labels. We find that: 1) using ImageNet weights in a
zero-shot image retrieval setting is only better than random guess by small margins; 2) all
in-domain pretrained CNN weights achieve much better retrieval performance than Image-
Net weights; and 3) our proposed ConVIRT pretraining achieves the best overall retrieval
results on all metrics. While Contrastive-Binary-Loss performs notably better than other
baselines in image-image retrieval, its text-image retrieval results are far from ConVIRT
pretraining. We conjecture that the lack of an explicit similarity-based loss function in
the Contrastive-Binary-Loss baseline results in misaligned representations in the image and
text space, leading to poor results in text-image retrieval.
To understand how well ConVIRT pretraining helps separate images from different ab-
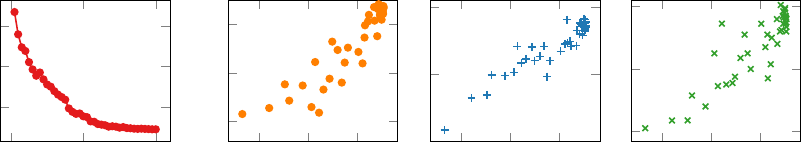
normality categories in its encoding space, in Figure 3 we present t-SNE plots (Maaten and
Hinton, 2008) of candidate images in the CheXpert 8x200 dataset for five selected categories,
from the ImageNet pretrained CNN encoder and the ConVIRT pretrained encoder. It is
worth noting that clustering images in our setting is much more challenging than that in
11

Contrastive Learning of Medical Visual Representations from Paired Images and Text
Image-Image Retrieval Text-Image Retrieval
Method Prec@5 Prec@10 Prec@50 Prec@5 Prec@10 Prec@50
Random 12.5 12.5 12.5 12.5 12.5 12.5
ImageNet 14.8 14.4 15.0 – – –
In-domain initialization methods
Caption-Transformer 29.8 28.0 23.0 – – –
Caption-LSTM 34.8 32.9 28.1 – – –
Contrastive-Binary-Loss 38.8 36.6 29.7 15.5 14.5 13.7
ConVIRT (Ours) 45.0 42.9 35.7 60.0 57.5 48.8
Fine-tuned
ConVIRT + CheXpert Supervised 56.8 56.3 48.9 – – –
Table 2: Zero-shot image-image and text-image retrieval results on the CheXpert 8×200
datasets. Random shows results from a random guess; ConVIRT + CheXpert Supervised
shows results from further fine-tuning the pretrained weights with supervised training data.
Text-image retrieval results are not obtained for some methods due to the lack of text
encoders.
(a) ImageNet Pretraining (b) ConVIRT Pretraining
Figure 3: t-SNE visualizations of encoded image representations from different pretraining
methods.
the general object classification setting due to the high inter-class similarity of the medical
images. Nevertheless we find that ConVIRT pretraining achieves a better clustering of the
images in the t-SNE plots.
6. Analysis and Discussion
Comparisons to Image-only Contrastive Learning. ConVIRT shows superior results
against baselines in evaluation, but an important question remains as to how it compares
against existing image-only contrastive learning methods. We study this by running two
popular such methods, SimCLR (Chen et al., 2020a) and MoCo v2 (Chen et al., 2020b),
on the same collection of images that we used in our pretraining. We present the results in
Table 3 and include training details in Appendix E. We find that compared to ImageNet
initialization, both contrastive methods lead to marginal to moderate improvements on the
12

Contrastive Learning of Medical Visual Representations from Paired Images and Text
RSNA CheXpert Image-Image
Method (Linear, 1%) (Linear, 1%) (Prec@10)
ImageNet 82.8 75.7 14.4
SimCLR (Chen et al., 2020a) 86.3 77.4 17.6
MoCo v2 (Chen et al., 2020b) 86.6 81.3 20.6
ConVIRT 90.7 85.9 42.9
Table 3: Comparisons of ConVIRT to image-only contrastive learning. For RSNA and
CheXpert we show the AUC under linear classification with 1% training data.
Figure 4: Saliency maps on sampled images for 4 abnormality categories in the CheXpert
dataset. For each image we present maps for ImageNet, SimCLR, MoCo v2 and our Con-
VIRT initializations. Ground truth regions that are indicative of the abnormalities are
shown as red boxes in the original images on the right, and are seen to most closely match
the regions found by ConVIRT.
classification and retrieval tasks. However, our training strategy substantially outperforms
both methods on all tasks, demonstrating its effective use of information from the paired
text data. This efficient use of data is critical to the healthcare domain because medical
data are often limited in size but come with paired text data and even user metadata.
13
Contrastive Learning of Medical Visual Representations from Paired Images and Text
0 100 200
2.4
2.6
2.8
(a) Pretraining Loss
−2.8 −2.6 −2.4
89.5
90
90.5
(b) RSNA Linear
(1%, AUC)
−2.8 −2.6 −2.4
25
35
45
(c) Image-image
(P@10)
−2.8 −2.6 −2.4
40
50
60
(d) Text-image
(P@10)
Figure 5: (a) shows pretraining validation loss at different epochs; (b)-(d) shows correlation
between the pretraining loss and the performance of three end tasks. For (a) the x-axis
shows the training epoch number, and for (b)-(d) the x-axis shows the negative value of the
pretraining loss (i.e., −L) on a held-out validation set.
To understand the representational difference that has led to this difference in per-
formance, for all four initialization methods, we visualize in Figure 4 the saliency maps
(Simonyan et al., 2014) corresponding to the correct class on sampled images from the
CheXpert dataset. Models for all initialization methods are trained with 1% CheXpert
training data under the linear classification setting (with pretrained CNN weights frozen).
We find that ImageNet pretraining has led to models that focus on trivial visual features
that are mostly irrelevant to the task, and that the model with ConVIRT pretrained weights
has focused on much more relevant areas than those with SimCLR and MoCo v2 pretrain-
ing, suggesting more effective representation learning. For example, for atelectasis, while
the ConVIRT model has correctly focused on the bottom of the lung regions, the SimCLR
model has much more scattered focus and the MoCo model has incorrectly focused on the
heart region.
Correlation Between Contrastive Loss and End Task Performance. To under-
stand the relation between a model’s performance on the ConVIRT pretraining task and
its performance on the downstream tasks, we ran an analysis where for every 5 epochs
during the pretraining, we transferred the pretrained checkpoint to the downstream tasks
and evaluate its performance. The pretraining was run for a total of 200 epochs, and 40
points were obtained with varying validation loss and end task results. Figure 5 presents
the results of the models’ validation loss on the pretraining task, and its achieved perfor-
mance on the RSNA 1% data linear evaluation and the two retrieval tasks. For all three
tasks, we find a clear positive correlation between the pretraining performance and the end
task performance. This corroborates that by learning with the ConVIRT objectives, the
image encoder learns gradually improved representations for the end tasks, and suggests
that further improvement on the pretraining task may have positive impact on the end task
performance.
Hyperparameter Analysis. We run experiments to study the impact of hyperparame-
ters, and have the following observations. First of all, similar to previous work on image-only
contrastive learning (Chen et al., 2020a; He et al., 2020), the pretraining results are most
14

Contrastive Learning of Medical Visual Representations from Paired Images and Text
RSNA Linear Image-Image Text-Image
Settings (1%, AUC) (Prec@10) (Prec@10)
ConVIRT (default) 90.7 42.9 57.5
τ = 0.01 90.7 40.5 21.0
τ = 1 89.6 25.0 31.0
bs = 16 90.3 40.0 55.8
bs = 128 90.3 39.3 50.3
linear proj. 90.6 40.8 55.8
Table 4: Evaluation results with different hyperparameters, for the RSNA 1% data linear
evaluation, image-image retrieval and text-image retrieval tasks. bs represents batch size
and linear proj. represents using linear projection layers for g
v
and g
u
. Our default model
uses τ = 0.1, bs = 32 and non-linear projections.
sensitive to the choice of the temperature value τ. As shown in Table 4, using a temperature
much lower than the ideal value (τ = 0.01) hurts the retrieval results, and a temperature
much larger (τ = 1) notably hurts the performance on all tasks. Second, unlike previous
work, changing batch size does not lead to substantial change in the classification results.
At last, replacing the non-linear projection heads in g
v
and g
u
with linear layers hurts the
retrieval results moderately, suggesting worse representations. However, this is again not
reflected notably in the RSNA classification results.
Limitations. This work mainly focuses on comparing ConVIRT against conventional
ImageNet initialization, image captioning-based initialization, and image-only contrastive
learning approaches including SimCLR and MoCo to demonstrate the data efficiency and
effectiveness of image-text pretraining. We did not compare our method against relevant
subsequent studies that extended ConVIRT, such as LoVT (M¨uller et al., 2021) or GloRIA
(Huang et al., 2021), mainly because such comparisons are included in these studies.
7. Conclusion
We presented ConVIRT, an unsupervised method for learning medical visual representa-
tions from paired descriptive text. Our method relies on contrasting the image repre-
sentations with the paired descriptive text via a bidirectional objective between the two
modalities. On 4 medical image classification tasks and 2 image retrieval tasks, ConVIRT
outperformed other strong in-domain initialization methods, and led to representations with
notably higher quality. Compared to ImageNet pretraining, ConVIRT is able to achieve
the same level of classification accuracy with an order of magnitude less labeled data. This
is especially critical for the healthcare domain where data sparsity is an important issue,
and the innovative cross-modality pretraining in ConVIRT is extensible to consider other
modalities of data in this domain. We thus hope that ConVIRT continues inspiring future
work that makes more efficient use of multi-modal data for medical image understanding.
15
Contrastive Learning of Medical Visual Representations from Paired Images and Text
References
Michael David Abr`amoff, Yiyue Lou, Ali Erginay, Warren Clarida, Ryan Amelon, James C
Folk, and Meindert Niemeijer. Improved automated detection of diabetic retinopathy on
a publicly available dataset through integration of deep learning. Investigative Ophthal-
mology & Visual Science, 57(13):5200–5206, 2016.
Emily Alsentzer, John Murphy, William Boag, Wei-Hung Weng, Di Jindi, Tristan Naumann,
and Matthew McDermott. Publicly available clinical BERT embeddings. In Proceedings
of the 2nd Clinical Natural Language Processing Workshop, 2019.
Shekoofeh Azizi, Basil Mustafa, Fiona Ryan, Zachary Beaver, Jan Freyberg, Jonathan
Deaton, Aaron Loh, Alan Karthikesalingam, Simon Kornblith, Ting Chen, et al. Big self-
supervised models advance medical image classification. In Proceedings of the IEEE/CVF
International Conference on Computer Vision (ICCV), 2021.
Ting Chen, Simon Kornblith, Mohammad Norouzi, and Geoffrey Hinton. A simple frame-
work for contrastive learning of visual representations. In International Conference on
Machine Learning (ICML), 2020a.
Xinlei Chen, Haoqi Fan, Ross Girshick, and Kaiming He. Improved baselines with momen-
tum contrastive learning. arXiv preprint arXiv:2003.04297, 2020b.
Marcella Cornia, Matteo Stefanini, Lorenzo Baraldi, and Rita Cucchiara. Meshed-memory
Transformer for image captioning. In Proceedings of the IEEE/CVF Conference on Com-
puter Vision and Pattern Recognition (CVPR), 2020.
Jeffrey De Fauw, Joseph R Ledsam, Bernardino Romera-Paredes, Stanislav Nikolov, Nenad
Tomasev, Sam Blackwell, Harry Askham, Xavier Glorot, Brendan O’Donoghue, Daniel
Visentin, et al. Clinically applicable deep learning for diagnosis and referral in retinal
disease. Nature Medicine, 24(9):1342–1350, 2018.
Dina Demner-Fushman, Marc D Kohli, Marc B Rosenman, Sonya E Shooshan, Laritza
Rodriguez, Sameer Antani, George R Thoma, and Clement J McDonald. Preparing a
collection of radiology examinations for distribution and retrieval. Journal of the Amer-
ican Medical Informatics Association, 23(2):304–310, 2016.
Karan Desai and Justin Johnson. VirTex: Learning visual representations from textual
annotations. In Proceedings of the IEEE Conference on Computer Vision and Pattern
Recognition (CVPR), 2021.
Jacob Devlin, Ming-Wei Chang, Kenton Lee, and Kristina Toutanova. BERT: Pre-training
of deep bidirectional transformers for language understanding. In Proceedings of the
2019 Conference of the North American Chapter of the Association for Computational
Linguistics: Human Language Technologies (NAACL-HLT), 2019.
Sedigheh Eslami, Gerard de Melo, and Christoph Meinel. Does CLIP benefit visual question
answering in the medical domain as much as it does in the general domain? arXiv preprint
arXiv:2112.13906, 2021.
16
Contrastive Learning of Medical Visual Representations from Paired Images and Text
Andre Esteva, Brett Kuprel, Roberto A Novoa, Justin Ko, Susan M Swetter, Helen M Blau,
and Sebastian Thrun. Dermatologist-level classification of skin cancer with deep neural
networks. Nature, 542(7639):115–118, 2017.
Jean-Bastien Grill, Florian Strub, Florent Altch´e, Corentin Tallec, Pierre Richemond, Elena
Buchatskaya, Carl Doersch, Bernardo Avila Pires, Zhaohan Guo, Mohammad Ghesh-
laghi Azar, Bilal Piot, Koray Kavukcuoglu, Remi Munos, and Michal Valko. Bootstrap
your own latent: A new approach to self-supervised learning. In Advances in Neural
Information Processing Systems, 2020.
Varun Gulshan, Lily Peng, Marc Coram, Martin C Stumpe, Derek Wu, Arunacha-
lam Narayanaswamy, Subhashini Venugopalan, Kasumi Widner, Tom Madams, Jorge
Cuadros, et al. Development and validation of a deep learning algorithm for detection of
diabetic retinopathy in retinal fundus photographs. JAMA, 316(22):2402–2410, 2016.
Tanmay Gupta, Arash Vahdat, Gal Chechik, Xiaodong Yang, Jan Kautz, and Derek Hoiem.
Contrastive learning for weakly supervised phrase grounding. In Proceedings of the 16th
European Conference on Computer Vision (ECCV), 2020.
Yan Han, Chongyan Chen, Ahmed Tewfik, Ying Ding, and Yifan Peng. Pneumonia detec-
tion on chest x-ray using radiomic features and contrastive learning. In 2021 IEEE 18th
International Symposium on Biomedical Imaging (ISBI). IEEE, 2021.
Kaiming He, Xiangyu Zhang, Shaoqing Ren, and Jian Sun. Deep residual learning for
image recognition. In Proceedings of the IEEE Conference on Computer Vision and
Pattern Recognition (CVPR), 2016.
Kaiming He, Haoqi Fan, Yuxin Wu, Saining Xie, and Ross Girshick. Momentum contrast for
unsupervised visual representation learning. In Proceedings of the IEEE/CVF Conference
on Computer Vision and Pattern Recognition (CVPR), 2020.
Lars Heiliger, Anjany Sekuboyina, Bjoern Menze, Jan Egger, and Jens Kleesiek. Beyond
medical imaging: A review of multimodal deep learning in radiology. TechRxiv preprint,
2022.
Olivier J H´enaff, Aravind Srinivas, Jeffrey De Fauw, Ali Razavi, Carl Doersch, SM Eslami,
and Aaron van den Oord. Data-efficient image recognition with contrastive predictive
coding. In International Conference on Machine Learning (ICML), 2020.
Shih-Cheng Huang, Liyue Shen, Matthew P Lungren, and Serena Yeung. GLoRIA: A mul-
timodal global-local representation learning framework for label-efficient medical image
recognition. In Proceedings of the IEEE/CVF International Conference on Computer
Vision (ICCV), 2021.
Gabriel Ilharco, Rowan Zellers, Ali Farhadi, and Hannaneh Hajishirzi. Probing contextual
language models for common ground with visual representations. In Proceedings of the
2021 Conference of the North American Chapter of the Association for Computational
Linguistics: Human Language Technologies (NAACL-HLT), 2021.
17
Contrastive Learning of Medical Visual Representations from Paired Images and Text
Jeremy Irvin, Pranav Rajpurkar, Michael Ko, Yifan Yu, Silviana Ciurea-Ilcus, Chris Chute,
Henrik Marklund, Behzad Haghgoo, Robyn Ball, Katie Shpanskaya, et al. CheXpert:
A large chest radiograph dataset with uncertainty labels and expert comparison. In
Proceedings of the AAAI Conference on Artificial Intelligence, 2019.
Chao Jia, Yinfei Yang, Ye Xia, Yi-Ting Chen, Zarana Parekh, Hieu Pham, Quoc Le, Yun-
Hsuan Sung, Zhen Li, and Tom Duerig. Scaling up visual and vision-language repre-
sentation learning with noisy text supervision. In Proceedings of the 38th International
Conference on Machine Learning, 2021.
Baoyu Jing, Pengtao Xie, and Eric Xing. On the automatic generation of medical imaging
reports. In Proceedings of the 56th Annual Meeting of the Association for Computational
Linguistics (ACL), 2018.
Alistair EW Johnson, Tom J Pollard, Seth J Berkowitz, Nathaniel R Greenbaum,
Matthew P Lungren, Chih-ying Deng, Roger G Mark, and Steven Horng. MIMIC-CXR,
a de-identified publicly available database of chest radiographs with free-text reports.
Scientific Data, 6, 2019.
Diederik P Kingma and Jimmy Ba. Adam: A method for stochastic optimization. In The
2015 International Conference for Learning Representations, 2015.
Ruizhi Liao, Daniel Moyer, Miriam Cha, Keegan Quigley, Seth Berkowitz, Steven Horng,
Polina Golland, and William M Wells. Multimodal representation learning via maxi-
mization of local mutual information. In International Conference on Medical Image
Computing and Computer-Assisted Intervention, 2021.
Tsung-Yi Lin, Michael Maire, Serge Belongie, James Hays, Pietro Perona, Deva Ramanan,
Piotr Doll´ar, and C Lawrence Zitnick. Microsoft COCO: Common objects in context. In
European Conference on Computer Vision (ECCV), 2014.
Guanxiong Liu, Tzu-Ming Harry Hsu, Matthew McDermott, Willie Boag, Wei-Hung Weng,
Peter Szolovits, and Marzyeh Ghassemi. Clinically accurate chest X-ray report generation.
In Machine Learning for Healthcare Conference, 2019.
Jiasen Lu, Dhruv Batra, Devi Parikh, and Stefan Lee. ViLBERT: Pretraining task-agnostic
visiolinguistic representations for vision-and-language tasks. In Advances in Neural In-
formation Processing Systems, 2019.
Laurens van der Maaten and Geoffrey Hinton. Visualizing data using t-SNE. Journal of
Machine Learning Research, 9(Nov):2579–2605, 2008.
Christopher D. Manning, Mihai Surdeanu, John Bauer, Jenny Finkel, Steven J. Bethard,
and David McClosky. The Stanford CoreNLP natural language processing toolkit. In
Association for Computational Linguistics (ACL) System Demonstrations, 2014.
Yasuhide Miura, Yuhao Zhang, Curtis P. Langlotz, and Dan Jurafsky. Improving factual
completeness and consistency of image-to-text radiology report generation. In Proceedings
of the 2021 Conference of the North American Chapter of the Association for Computa-
tional Linguistics: Human Language Technologies (NAACL-HLT), 2021.
18
Contrastive Learning of Medical Visual Representations from Paired Images and Text
Philip M¨uller, Georgios Kaissis, Congyu Zou, and Daniel R¨uckert. Joint learning of localized
representations from medical images and reports. arXiv preprint arXiv:2112.02889, 2021.
Aaron van den Oord, Yazhe Li, and Oriol Vinyals. Representation learning with contrastive
predictive coding. arXiv preprint arXiv:1807.03748, 2018.
Alec Radford, Jong Wook Kim, Chris Hallacy, Aditya Ramesh, Gabriel Goh, Sandhini
Agarwal, Girish Sastry, Amanda Askell, Pamela Mishkin, Jack Clark, et al. Learning
transferable visual models from natural language supervision. In International Conference
on Machine Learning, 2021.
Maithra Raghu, Chiyuan Zhang, Jon Kleinberg, and Samy Bengio. Transfusion: Under-
standing transfer learning for medical imaging. In Advances in Neural Information Pro-
cessing Systems, 2019.
Pranav Rajpurkar, Jeremy Irvin, Aarti Bagul, Daisy Ding, Tony Duan, Hershel Mehta,
Brandon Yang, Kaylie Zhu, Dillon Laird, Robyn L Ball, et al. MURA: Large dataset
for abnormality detection in musculoskeletal radiographs. In 1st Conference on Medical
Imaging with Deep Learning (MIDL), 2018a.
Pranav Rajpurkar, Jeremy Irvin, Robyn L Ball, Kaylie Zhu, Brandon Yang, Hershel Mehta,
Tony Duan, Daisy Ding, Aarti Bagul, Curtis P Langlotz, et al. Deep learning for chest ra-
diograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing
radiologists. PLoS Medicine, 15(11):e1002686, 2018b.
Olga Russakovsky, Jia Deng, Hao Su, Jonathan Krause, Sanjeev Satheesh, Sean Ma, Zhi-
heng Huang, Andrej Karpathy, Aditya Khosla, Michael Bernstein, et al. ImageNet large
scale visual recognition challenge. International Journal of Computer Vision, 115(3):
211–252, 2015.
Mert Bulent Sariyildiz, Julien Perez, and Diane Larlus. Learning visual representations
with caption annotations. In Proceedings of the 16th European Conference on Computer
Vision (ECCV), 2020.
George Shih, Carol C Wu, Safwan S Halabi, Marc D Kohli, Luciano M Prevedello, Tessa S
Cook, Arjun Sharma, Judith K Amorosa, Veronica Arteaga, Maya Galperin-Aizenberg,
et al. Augmenting the National Institutes of Health chest radiograph dataset with expert
annotations of possible pneumonia. Radiology: Artificial Intelligence, 1(1):e180041, 2019.
Karen Simonyan, Andrea Vedaldi, and Andrew Zisserman. Deep inside convolutional net-
works: Visualising image classification models and saliency maps. In ICLR Workshop,
2014.
Hari Sowrirajan, Jingbo Yang, Andrew Y Ng, and Pranav Rajpurkar. MoCo pretraining
improves representation and transferability of chest X-ray models. In Medical Imaging
with Deep Learning, pages 728–744. PMLR, 2021.
Weijie Su, Xizhou Zhu, Yue Cao, Bin Li, Lewei Lu, Furu Wei, and Jifeng Dai. VL-BERT:
Pre-training of generic visual-linguistic representations. In International Conference on
Learning Representations (ICLR), 2020.
19
Contrastive Learning of Medical Visual Representations from Paired Images and Text
Hao Tan and Mohit Bansal. LXMERT: Learning cross-modality encoder representations
from transformers. In Proceedings of the 2019 Conference on Empirical Methods in Natu-
ral Language Processing and the 9th International Joint Conference on Natural Language
Processing (EMNLP-IJCNLP), 2019.
Ramakrishna Vedantam, C Lawrence Zitnick, and Devi Parikh. CIDEr: Consensus-based
image description evaluation. In Proceedings of the IEEE Conference on Computer Vision
and Pattern Recognition (CVPR), 2015.
Yen Nhi Truong Vu, Richard Wang, Niranjan Balachandar, Can Liu, Andrew Y Ng, and
Pranav Rajpurkar. MedAug: Contrastive learning leveraging patient metadata improves
representations for chest x-ray interpretation. In Machine Learning for Healthcare Con-
ference, 2021.
Linda Wang and Alexander Wong. COVID-Net: A tailored deep convolutional neural
network design for detection of COVID-19 cases from chest X-ray images. arXiv preprint
arXiv:2003.09871, 2020.
Xiaosong Wang, Yifan Peng, Le Lu, Zhiyong Lu, Mohammadhadi Bagheri, and Ronald M
Summers. ChestX-ray8: Hospital-scale chest X-ray database and benchmarks on weakly-
supervised classification and localization of common thorax diseases. In Proceedings of
the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), 2017.
Xiaosong Wang, Yifan Peng, Le Lu, Zhiyong Lu, and Ronald M Summers. TieNet: Text-
image embedding network for common thorax disease classification and reporting in chest
X-rays. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recog-
nition (CVPR), 2018.
Xiaosong Wang, Ziyue Xu, Leo Tam, Dong Yang, and Daguang Xu. Self-supervised image-
text pre-training with mixed data in chest x-rays. arXiv preprint arXiv:2103.16022, 2021.
Thomas Wolf, Lysandre Debut, Victor Sanh, Julien Chaumond, Clement Delangue, An-
thony Moi, Pierric Cistac, Tim Rault, Remi Louf, Morgan Funtowicz, Joe Davison,
Sam Shleifer, Patrick von Platen, Clara Ma, Yacine Jernite, Julien Plu, Canwen Xu,
Teven Le Scao, Sylvain Gugger, Mariama Drame, Quentin Lhoest, and Alexander Rush.
Transformers: State-of-the-art natural language processing. In Proceedings of the 2020
Conference on Empirical Methods in Natural Language Processing (EMNLP): System
Demonstrations, 2020.
Kelvin Xu, Jimmy Ba, Ryan Kiros, Kyunghyun Cho, Aaron Courville, Ruslan Salakhudinov,
Rich Zemel, and Yoshua Bengio. Show, attend and tell: Neural image caption generation
with visual attention. In International Conference on Machine Learning (ICML), 2015.
Chengxi Zang and Fei Wang. Scehr: Supervised contrastive learning for clinical risk pre-
diction using electronic health records. arXiv preprint arXiv:2110.04943, 2021.
20

Contrastive Learning of Medical Visual Representations from Paired Images and Text
Appendix A. Model Implementation and Pretraining Details
Dataset Preprocessing. For the MIMIC-CXR chest radiograph dataset, we use the
publicly available JPG version of it.
2
For both the MIMIC-CXR chest dataset and the
Rhode Island Hospital bone image datasets, we resize the image files to have a size of 256
on the larger side. For the textual radiology report data, we first tokenize all reports with
the default English tokenizer in version 4.0.0 of the CoreNLP library (Manning et al., 2014).
Next, we keep only the Findings and Impression sections and remove all other sections. We
remove all image-text pairings from the dataset where the text section is empty or has less
than 3 tokens. This preprocessing procedure gives us about 217k total image-text pairs
for pretraining our chest image encoder and 48k total pairs for pretraining our bone image
encoder.
Image and Text Encoders. For the image encoder, we use the standard ResNet50
implementation provided by the torchvision library. For the text encoder, we use the BERT
base encoder offered by the Transformers library (Wolf et al., 2020) and initialize it with
the ClinicalBERT model (Alsentzer et al., 2019) pretrained on the MIMIC clinical notes.
We also experimented with training a specialized BERT encoder on a large collection of
radiology notes but found that it made no substantial difference in the pretraining results.
At pretraining time we freeze the embeddings and the first 6 layers of this BERT encoder,
and only fine-tune the last 6 layers for our contrastive task.
Other Hyperparameters. For contrastive learning, we use projection layers with an
output dimension d = 512, a temperature value τ = 0.1, a loss weight λ = 0.75. These
hyperparameter settings are obtained by comparing the linear evaluation validation scores
on the RSNA image classification task with the pretrained ResNet50 weights. For the
image transformation family T , we adopt the implementations offered by the torchvision
library.
3
We apply random cropping with a ratio sampled from [0.6, 1.0]; horizontal flipping
with p = 0.5; affine transformation with a degree sampled from [−20, 20], max horizontal
and vertical translation fractions of 0.1, and a scaling factor sampled from [0.95, 1.05];
color jittering with brightness and contrast adjustment ratios sampled from [0.6, 1.4]; and
Gaussian blur with σ ∈ [0.1, 3.0]. All images are resized to 224×224 after the transformation
t
v
is applied. Limited by computational resources, we arrive at these image transformation
parameters via preliminary experiments rather than a systematic search.
Pretraining Details. At pretraining time, for each dataset, we randomly sample 5k
image-text pairs to form a held-out validation set. We we use the Adam optimizer (Kingma
and Ba, 2015) with an initial learning rate of 1e-4 and weight decay of 1e-6. We initialize the
image encoder with ImageNet pretrained weights at the beginning of pretraining, and use a
fixed batch size of 32. We calculate the validation loss every 5000 steps, and if the validation
loss does not decrease after 5 straight evaluation runs, we anneal the learning rate by a factor
of 0.5. We stop pretraining after 200 evaluation runs, and save the model checkpoint that
achieves the lowest validation loss. For efficiency, we employ mixed-precision training, and
for reference, the whole pretraining run on the MIMIC-CXR dataset took about 3 days on
a single Titan RTX GPU card.
2. https://physionet.org/content/mimic-cxr-jpg/2.0.0/
3. https://github.com/pytorch/vision
21

Contrastive Learning of Medical Visual Representations from Paired Images and Text
Appendix B. Image Classification Experiments
We prepared and used the 4 image classification datasets following the procedures below:
1. RSNA Pneumonia Detection (Wang et al., 2017; Shih et al., 2019): we used the orig-
inal version of this dataset available at its Kaggle page,
4
which contains 25184/1500/3000
annotated images in its training/validation/test sets, respectively.
2. CheXpert image classification (Irvin et al., 2019): we downloaded the original version
of this dataset from its official website.
5
Since the original expert-labeled test set of this
dataset is hidden and not included as part of the release, we instead followed Raghu et al.
(2019) and used the original expert-labeled validation set as our test set, and randomly
sampled 5000 images from the original training set for validation purpose. The resulting
dataset contains 218414/5000/234 images in each split.
3. COVIDx image classification (Wang and Wong, 2020): we prepared this dataset fol-
lowing the scripts provided by its authors.
6
We used the version 4 of this dataset, the
latest version at the time of this work. We additionally randomly sampled 300 images
from the training set for validation, resulting in a dataset with 13598/300/300 images in
each split.
4. MURA bony abnormality detection (Rajpurkar et al., 2018a): we downloaded the orig-
inal version of this dataset from its website.
7
Similar to the CheXpert dataset, we again
used the original validation set as our test set, and randomly sampled 10% images from
the training set for validation, resulting in a dataset with 33078/3730/3197 images in
each split. Different from the other 3 datasets, the MURA dataset uses patient-level
evaluation, meaning that the prediction results from different images of the same patient
needs to be aggregated to produce a final prediction for the patient, which is then scored
against the gold patient label. We therefore followed Rajpurkar et al. (2018a) and at
test time aggregated result for a patient by averaging the predicted probabilities from
multiple images.
Classification Model Training Details. For all models that require ImageNet pre-
trained initialization, we use the pretrained weights from torchvision, which achieves an
ImageNet top-5 error rate of 7.13%. For all datasets, we first zero-pad the input image to
be square, and then resize it to be 224×224. For training, we use the Adam optimizer with
an initial learning rate of 1e-3 for the COVIDx task and 1e-4 for the other three tasks. We
additionally apply a weight decay of 1e-6 and a dropout before the last classification layer
with p = 0.2 in all tasks. All classification models are trained with a batch size of 64. In
the fine-tuning evaluation setting, we first “warmup” the classification head by freezing the
CNN weights and only training the classification head with a learning rate of 1e-3 for 200
steps, after which we unfreeze the CNN weights and fine-tune the entire network together.
Validation score is obtained after each epoch of training and we anneal the learning rate
4. https://www.kaggle.com/c/rsna-pneumonia-detection-challenge
5. https://stanfordmlgroup.github.io/competitions/chexpert/
6. https://github.com/lindawangg/COVID-Net
7. https://stanfordmlgroup.github.io/competitions/mura/
22

Contrastive Learning of Medical Visual Representations from Paired Images and Text
by a factor of 0.5 if the validation score is not improved after 3 epochs. The training is
stopped after no validation improvement is observed for 10 straight epochs, at which point
the model checkpoint with the highest validation score is evaluated on the test set.
Image Category Example Textual Query
Atelectasis Platelike opacity likely represents atelectasis.
Cardiomegaly The cardiac silhouette is enlarged.
Edema The presence of hazy opacity suggests interstitial pulmonary edema.
Fracture A cortical step off indicates the presence of a fracture.
Pleural Effusion The pleural space is partially filled with fluid.
Pneumonia A pulmonary opacity with ill defined borders likely represents pneumonia.
Pneumothorax A medial pneumothorax is present adjacent to the heart.
No Finding No clinically significant radiographic abnormalities.
Table 5: Example textual queries for each of the 8 categories in the text-image retrieval
task.
Appendix C. Image-image Retrieval Dataset Collection
We create the CheXpert 8×200 Retrieval Dataset with 8 different abnormality categories
commonly found in Chest radiograph images, including atelectasis, cardiomegaly, edema,
fracture, pleural effusion, pneumonia, pneumothorax and a special no finding category indi-
cating that no obvious abnormality is found in the image. We create the dataset by reusing
existing rule-labeled annotations in the CheXpert dataset (Irvin et al., 2019) and additional
expert annotations. To create the candidate images for a category label ℓ, we go through
all images in the CheXpert training set, and keep an image as a candidate image if only its
label for ℓ is positive and all other categories negative. We only include images with this
“exclusive positivity” as candidate images, mainly to avoid confounding results between
categories in retrieval evaluation.
To create the query images for a category ℓ, we again first pre-select 50 exclusively
positive images for this category in the CheXpert training set (with all candidate images
excluded). Next, we ask a board-certified radiologist to examine each of the 50 images,
and exclude images that: 1) might indicate additional abnormalities other than ℓ, 2) have
uncommon color or contrast distortions in the image, or 3) are not well posed during the
capture of the image. This procedure is mainly to avoid including query images that have
uncommon features and may therefore bias the retrieval evaluation results. At the end, we
aggregate the annotation results from the radiologist and keep 10 query images for each
abnormality category.
Appendix D. Text-image Retrieval Dataset Collection
For the text-image retrieval dataset, we first reuse all candidate images from the CheXpert
8×200 image-image retrieval dataset described above, with 200 images for each of 8 cate-
23
Contrastive Learning of Medical Visual Representations from Paired Images and Text
gories. To create the textual queries for each abnormality category, we ask a board-certified
radiologist to write at least 5 different sentences that he will use to describe this abnormal-
ity in radiology reporting. We additionally set the following requirements: 1) the sentences
must describe the category with no ambiguity and must not include other categories; 2)
the sentences must be diverse from each other; and 3) the sentences should not include
very specific anatomic locations or rare clinical observations. At the end, we aggregate the
results and keep 5 textual queries for each abnormality category. For reference, we present
example textual queries in Table 5.
Appendix E. Experiments on Image-Only Contrastive Learning Methods
We run experiments with two popular image-only contrastive visual representation learning
methods: SimCLR (Chen et al., 2020a) and MoCo v2 (Chen et al., 2020b). For a fair
comparison, in both experiments we use the exact same set of images from the MIMIC-
CXR dataset that we use in the pretraining of our method and the baselines. Our settings
for each method are:
• SimCLR: We use the open PyTorch implementation available at https://github.com/
sthalles/SimCLR. For image encoder we use ResNet50. We use cosine similarity in
the loss function, set the temperature value to 0.1 and set the output dimension to
128. We use the default image augmentation functions in the paper except for the color
jittering transformation where we set the saturation and hue adjustment to 0 due to the
monochrome nature of our medical images. For training, we use the Adam optimizer with
an initial learning rate of 3e-4 and weight decay of 1e-4. We set batch size to 128 and
run training on a single GPU card for 100 epochs, as we find that increasing the batch
size or number of epochs does not lead to improved results. We use the default settings
for all other parameters.
• MoCo v2: We use the authors’ PyTorch implementation available at https://github.
com/facebookresearch/moco. For image encoder we use ResNet50. We follow the de-
fault MoCo v2 setting and use a temperature value of 0.07 and an output dimension of
128. Similarly, we adopt the default image augmentation functions except for the color
jittering transformation where we set the saturation and hue adjustment to 0. For train-
ing, we use the SGD optimizer with a learning rate of 0.0075 and weight decay of 1e-4.
We use a batch size of 64 and a queue size of 4096, and run parallel training on two
GPU cards for 100 epochs, as we find that further increasing the batch size or number of
epochs does not lead to improved results. During training, we anneal the learning rate
by a factor of 0.1 at the 60th and 80th epochs.
24
