
FORMS VERSION H SERIES
Released: August 5
th
, 2023
MULTI - PROJECT INSTRUCTIONS FOR
NIH AND OTHER PHS AGENCIES
SF424 (R&R) APPLICATION PACKAGES
Guidance developed and maintained by NIH for preparing and
submitting applications via Grants.gov to NIH and other PHS
agencies using the SF424 (R&R)

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
TABLE OF CONTENTS
TABLE OF CONTENTS
TABLE OF CONTENTS 2
M.100 - How to Use the Application Instructions 3
M.110 - Application Process 6
M.120 - Significant Changes 10
M.130 - Program Overview 13
M.200 - SF 424 (R&R) Form 18
M.210 - PHS 398 Cover Page Supplement Form 33
M.220 - R&R Other Project Information Form 38
M.230 - Project/Performance Site Location(s) Form 49
M.240 - R&RSenior/Key Person Profile (Expanded) Form 53
M.300 - R&R Budget Form 64
M.310 - R&R Subaward Budget Attachment(s) Form 81
M.330 - PHS 398 Training Budget Form 84
M.340 - PHS 398 Training Subaward Budget Attachment(s) Form 90
M.350 - PHS Additional Indirect Costs Form 93
M.400 - PHS 398 Research Plan Form 96
M.410 - PHS 398 Career Development Award Supplemental Form 113
M.420 - PHS 398 Research Training Program Plan Form 138
M.500 - PHS Human Subjects and Clinical Trials Information 156
M.600 - PHS Assignment Request Form 192
Form Screenshots i
M - 2

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.100 - How to Use the Application Instructions
M.100 - How to Use the Application
Instructions
Use these application instructions to fill out the forms that are posted in your funding
opportunity announcement.
View the How to Apply Video Tutorials.
Quick Links
Step 1. Become familiar with the application process
Step 2. Use these instructions, together with the forms and information in the funding oppor-
tunity announcement, to complete your application
Step 3. Choose an application instruction format
Step 4. Complete the appropriate forms
Step 5. Stay informed of policy changes and updates
Step 6. Understand what data NIH makes public
Helpful Links
The information on the following pages may be useful in the application process
l
NIH Grants & Funding Glossary
l
Grants Policy Statement
l
NIH Guide to Grants and Contracts
l
Frequently Asked Questions
Step 1. Become familiar with the application process.
Understanding the application process is critical to successfully submitting your application.
Use the M.110 - Application Process section of these instructions to learn the importance of
completing required registrations before submission, how to submit and track your application,
where to find page limits and formatting requirements, and more information about the
application process.
M - 3

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.100 - How to Use the Application Instructions
Step 2. Use these instructions, together with the forms and information
found in the funding opportunity announcement, to complete your
application.
The funding opportunity announcement (FOA)will include specific instructions and the forms
needed for your application submission.
Remember that the FOA instructions always supersede these application instructions.
Step 3. Choose an application instruction format.
Do you know your activity code, but don’t know which application instructions to use? Refer to
NIH’s table on Determine the Correct Application Instructions for Your Activity Code to identify the
set of application instructions applies to your grant program.
Comprehensive Instructions Program-Specific Instructions
Use the General (G) instructions, available in
both
HTML
and
PDF
format, to complete
the application forms for any type of grant
program.
Take advantage of the filtered PDFs to view
specific application instructions for:
l
Research (R)
l
Career Development (K)
l
Training (T)
l
Fellowship (F)
l
Multi-project (M)
l
SBIR/STTR (B)
Step 4. Complete the appropriate forms.
Unless otherwise specified in the FOA, follow the standard instruction, as well as any additional
program-specific instructions for each form in your application.
Program-specific instructions are presented in gray call-out boxes that are color coded throughout
the application instructions. Consult the M.130 - Program Overview section for context for
program specific instructions.
IMPORTANT: Do Not Include Personal Identifiable Information (PII) Or Protected
Health Information (PHI) In the Application
Sensitive PII (e.g., Social Security Number, personal financial information, Alien Registration
Number) and PHI (e.g., personal medical conditions) require strict handling due to the increased
risk to an individual if the data is compromised. Documents containing sensitive PII or PHI must
not be included in the application.
M - 4

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.100 - How to Use the Application Instructions
NIH GRANTS ONLY: Additional information on this topic can be found in this FAQ or by
contacting your IC Privacy Coordinator.
Step 5. Stay informed of policy changes and updates.
l
Refer to the M.120 - Significant Changes section for the most recent changes to these
application instructions.
l
Review Notices of NIH Policy Changes since the posting of the Application Guide.
Step 6. Understand what data NIH makes public.
Information submitted as part of the application will be used by reviewers to evaluate the scientific
merit of the application and by NIH staff to make the grant award and monitor the grant after
award. The exception to this is the M.600 - PHS Assignment Request Form, which is only seen by
staff in the Division of Receipt and Referral (DRR), Center for Scientific Review (CSR).
If the application is funded, the following fields will be made available to the public through the
NIH Research Portfolio Online Reporting Tool (RePORTER) and will become public information:
l
Name of Project Director/Principal Investigator (PD/PI), to also include Project Leaders on sub-
projects to multi-project projects
l
PD/PI title
l
PD/PI email address
l
Organizational name
l
Institutional address
l
Project summary/abstract
l
Public health relevance statement
In addition, key elements related to ongoing funded projects will be made available to the public,
including those listed in the data dictionary at ExPORTER. Additional elements may be made
available after announcements through the NIH Guide for Grants and Contracts, a weekly
electronic publication that is available on NIH’s Funding page, or additions to the NIH Grants
Policy Statement, as needed.
M - 5

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.110 - Application Process
M.110 - Application Process
Understanding the application process is critical to successfully submitting your application.
Use this section of this guide to learn the importance of completing required registrations
before submission; how to submit and track your application; where to find information about
page limits, formatting requirements, due dates, and submission policies; and more
information about the application process. This application process information is also
available on our How to Apply – Application Guide page.
Quick Links
Prepare to Apply and Register
Write Application
Submit
Related Resources
Prepare to Apply and Register
Systems and Roles
Learn about the main systems involved in application submission and the role you and your
colleagues play in the submission process. The main systems are Grants.gov, eRA Commons, and
ASSIST.
Register
Determine your registration status. Organizations, organizational representatives, investigators,
and others need to register in multiple federal systems in order to for you to submit a grant
application. Registration can take six weeks or more to complete. Start today! See NIH’s
Registration website.
Understand Funding Opportunities
Identify the right funding opportunity announcement (FOA) for your research and learn about key
information you will find in the FOA.
Types of Applications
Are you submitting a new, renewal, revision, or resubmission application? Learn about the
different types of applications and special submission requirements.
Submission Options
Determine which system is most convenient for your application submission: NIH’s ASSIST web-
based application submission system, Grants.gov Workspace, or, if applicable, your organization’s
own submission system.
M - 6

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.110 - Application Process
Obtain Software
Applicants must have the free Adobe Reader software, a PDF generator, and a web browser to
submit an application. Learn which versions are compatible with our systems.
Write Application
Write Your Application
Read tips for developing a strong application that helps reviewers evaluate its science and merit.
Develop Your Budget
Learn about the kinds of costs you may include in your budget submission, the difference between
modular and detailed budgets, and more about how to develop your budget.
Format Attachments
Follow these requirements for preparing the documents you attach to your application.
Requirements include criteria for the PDF files, fonts, margins, headers and footers, paper size,
citations, formatting pages, use of hyperlinks and URLs, etc.
Rules for Text Fields
Learn the rules for form text fields – allowable characters, cutting and pasting, character limits, and
formatting.
Page Limits
Follow the page limits specified in this table for your specific grant program, unless otherwise
specified in the FOA.
Data Tables
Find instructions, blank data tables, and samples to use with institutional research training
applications.
Reference Letters
Some types of programs, such as fellowships and some career development awards, require the
submission of reference letters by the referee. Learn about selecting a referee and find instructions
for submission.
Biosketches
Biosketches are required in both competing applications and progress reports. Find instructions,
blank format pages, limitation on use of hyperlinks and URLs, and sample biosketches.
Submit
Submit, Track and View
Learn how to submit your application, and about your responsibility for tracking your application
and viewing the application image in the eRA Commons before the application deadline. If you
can’t view your application in eRA Commons, we can’t review it.
M - 7

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.110 - Application Process
How We Check for Completeness
Your application will be checked at Grants.gov, by eRA systems, and by federal staff before it is
referred for review.
Changed/Corrected Applications
You will need to submit a changed/corrected application to correct issues that either you or our
systems find with your application. Learn how and when you may submit a changed/corrected
application.
Related Resources
Due Dates and Policies
Due Dates
View standard due dates for competing applications. The FOA will identify whether to follow
standard due dates or whether to follow an alternative due date.
Submission Policies
Learn the nuances of application submission policies, including when late applications might be
allowed, what to do if due dates fall on a weekend or holiday, whether we allow post-submission
materials, how to document system issues, the rules around resubmission applications, etc.
Dealing with System Issues
Are you experiencing system issues with ASSIST, Grants.gov, System for Award Management
(SAM), or the eRA Commons that you believe threaten your ability to submit on time? NIH will not
penalize applicants who experience confirmed issues with federal systems that are beyond their
control. You must report the problem before the submission deadline.
After Submission
Receipt and Referral
Understand how and when applications are given an application identification number and
assigned to a review group and an NIH Institute or Center (IC) for possible funding.
Peer Review
Learn about our two phase peer review process, including initial peer review, Council review,
review criteria, scoring, and summary statements.
Pre-award Process
Learn what happens between peer review and award for applications that have been deemed
highly meritorious in the scientific peer review process. Be ready: if you received a great score in
peer review, you’ll have to submit Just-in-Time information.
Post award Monitoring andReporting
If you receive a grant from the NIH, you will need a lot of information to be a successful steward of
federal funds. This page provides a brief overview of grantee monitoring and reporting
requirements.
M - 8

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.110 - Application Process
Resources
Annotated Form Sets
These handy documents are a great visual resource for understanding many of the validation
checks we will run against your submitted application.
Contacting NIH Staff
NIH staff is here to help. We strongly encourage NIH applicants and grantees to communicate
with us throughout the grant life cycle. Understanding the roles of NIH staff can help you contact
the right person at each phase of the application and award process.
Contacting Staff at Other PHS Agencies
Applicants are strongly encouraged to communicate with agency staff throughout the entire
application review and awards process.
Systems
ASSIST
eRA Commons
Grants.gov
Information Collection
Authorization
The PHS Act establishes the authority with which NIH and other PHS agencies award grants and
collect information related to grant awards.
Paperwork Burden
The paperwork burden provides the estimated time for completing a grant application.
Collection of Personal Demographic Data
NIH collects personal data through the eRA Commons Personal Profile. The data is confidential
and is maintained under the Privacy Act record system.
M - 9

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.120 - Significant Changes
M.120 - Significant Changes
The Application Instructions are updated and released 2-3 times per year as needed.
Additionally, minor revisions may be made outside of these releases.
This section details all significant changes and revisions made to the instructions since the last
major release.
Within the instructions, new instructions will be marked with this symbol.
In the web version, use your mouse to hover over the icon to read an explanation of the
change.
In a PDF version, this symbol will be visible but will not display hover text. For more
information, see the explanation in the Significant Changes section below.
Release Notes - August 5, 2023
Program Overview - Small Business Innovation Research (SBIR) and Small Business Technology
Transfer (STTR)
Added new “Disclosure Requirements Regarding Ties to Foreign Countries” and “Denial of
Awards” subsections outlining requirements related to the implementation of NIH SBIR and STTR
Foreign Disclosure Pre-award Requirements and the HHS Due Diligence Program, applicable for
competing applications submitted for due dates on or after September 5, 2023.
R&R Other Project Information Form
Updated Additional Instructions for SBIR/STTR for the content of 10. Facilities & Other Resources
for applications submitted for due dates on or after September 5, 2023.
R&R Budget and associated R&R Subaward Budget Attachment(s) Form
Updated special instructions for applicants submitting a Data Management and Sharing (DMS)
Plan within the following sections for applications submitted for due dates on or after October 5,
2023:
Under “Who should use the R&R Budget Form?” “Additional instructions for Multi-Project”
B. Other Personnel. Other Direct Costs “8-17 Other”
L. Budget Justification
PHS 398 Modular Budget Form
Updated instructions for the “Additional Narrative Justification” Budget Justification attachment
content and special instructions for applicants submitting Data Management and Sharing (DMS)
Plans.
M - 10

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.120 - Significant Changes
Release Notes - October 25, 2022
How to Use the Application Instructions
l
Added reminder to not include personal identifiable information (PII) or protected health
information (PHI) in the application.
SF-424 Research and Related (R&R)Form Changes
R&RBudget and associated R&RSubaward Budget Attachment(s) Form
l
Added special instructions for applicants submitting a Data Management and Sharing
(DMS)Plan within the following sections:
l
Under “Who should use the R&R Budget Form?” “Additional instructions for Multi-
Project”
l
B. Other Personnel. Other Direct Costs “8-17 Other”
l
L. Budget Justification
Forms-H Changes
FORMS-H application packages incorporate the latest versions of the PHS forms managed by
NIH (OMB Number: 0925-0001 and 0925-0770, Expiration Date: 01/31/2026).
PHS 398 Modular Budget Form
l
Added special instructions for applicants submitting Data Management and Sharing (DMS)
Plans.
PHS 398 Research Plan Form
l
Added new item 11. Other Plan(s) and added instructions for applicants submitting Data
Management and Sharing (DMS) Plans.
l
Renumbered form fields.
l
Updated instructions for section 10, Resource Sharing Plan(s) to remove instructions for the
Data Sharing Plan and Genomic Data Sharing (GDS). The DMS Plan will now be attached in the
new item 11, Other Plan(s).
l
Clarified instructions for renewal or resubmission applications involving changes between
single PD/PI to or from multiple PD/PIs.
PHS 398 Career Development Award Supplemental Form
l
Added new item 17. Other Plan(s) and added instructions for applicants submitting Data
Management and Sharing (DMS) Plans.
l
Renumbered form fields.
M - 11

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.120 - Significant Changes
l
Updated instructions for 16. Resource Sharing Plan(s) to remove instructions for the Data
Sharing Plan and Genomic Data Sharing (GDS). The DMS Plan will now be attached in the new
item 17. Other Plan(s).
PHS 398 Research Training Program Plan Form
l
Added new item 13. Other Plan(s) for potential future use with other plans.
l
Renumbered form fields.
l
Clarified instructions for renewal or resubmission applications involving changes between
single PD/PI to or from multiple PD/PIs.
PHS Fellowship Supplemental Form
l
Added new item 17. Other Plan(s) for potential future use with other plans.
l
Renumbered form fields.
l
Updated instructions for 16. Resource Sharing Plan(s) to remove instructions for the Data
Sharing Plan and Genomic Data Sharing (GDS).
Form Screenshots
l
Updated form screenshots.
M - 12
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.130 - Program Overview
M.130 - Program Overview
Quick Links
Multi-project Applications ("M" Series).
Multi-project Applications ("M"Series)
A multi-project application is a single submission with multiple, interrelated components that
share a common focus or objective.
A component is a distinct, reviewable part of a multi-project application for which there is a
business need to gather detailed information as defined in a particular funding opportunity
announcement (FOA). Components typically include general information (component
organization, project period, project title, etc.), information about performance sites, information
about proposed work to be accomplished, and a budget.
Additional Instructions for Multi-project:
Additional multi-project instructions will be denoted by a gray call-out box with red
color coding and with the heading “Additional Instructions for Multi-project”
throughout these application instructions.
Although multi-project applications use the same forms used for single-project applications, there
are some differences in the way multi-project applications are structured. Every multi-project
application includes:
l
A Single Overall Component: The Overall Component describes the entire application and
provides an overview of how each of the other components fit together.
l
One or more Other Component Types: Other Component types (e.g., Admin Core, Project
Core) will vary by opportunity and will be specified in the FOA.
l
Summaries: Information is automatically compiled from the data provided by the applicant in
the individual components and included as part of the Overall Component in the agency-
assembled application to help reviewers and staff work with the application. The following
summaries are generated:
l
Component
l
Performance Sites
l
Human Subjects - Clinical Trials – Vertebrate Animals- hESC
l
Human Embryonic Stem Cell Lines
l
Budget
l
Program Income
l
Senior/Key Personnel
l
Biosketches
M - 13

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.130 - Program Overview
For information on how your application will be automatically assembled for review and funding
consideration after submission, see the How eRA Assembles Multi-project Applications file.
Before Applying:
1. Become familiar with Activity Code: Applicants should become familiar with the
activity code(s) for which support is being requested. A comprehensive list of all activity
codes, with their descriptions, is available on the Activity Codes Search Results website.
2. Refer to your specific FOA: Refer to your specific FOA for specific information
associated with the award mechanism, including special application instructions.
l
The FOA will specify the types of Other Components that should be used when
preparing the application, whether each component is optional or required, and
any restrictions on the number of times each component can be included in an
application.
3. Contact Awarding Component: Applicants are encouraged to consult with the NIH
Scientific/Research contact of the appropriate awarding component prior to submitting
an application, as eligibility criteria, support levels, and availability of awards may vary
among NIH Institutes or Centers and other PHS agencies.
Collaborating with Other Organizations
Multi-project applications often include a number of collaborating organizations in addition to the
applicant organization. The applicant organization always has primary responsibility for and leads
the Overall Component. A collaborating organization may be responsible for a small part of a
component or have lead responsibility for an entire Other Component within the application.
Depending on the role of the collaborating organization(s) in the project, there are two
approaches to structuring a component:
A. Collaborating Organization as the Lead of a Component:
When the bulk of the leadership and work on a component (other than the Overall
Component) is performed by a collaborating organization, then that organization can be set
up as the lead organization for that component. All the component forms (including the SF
424 R&R Form and the R&R Budget Form) are completed using the collaborating
organization’s information. On the R&R Budget Form, use the Budget Type “Project” to
identify it as the primary budget for the component and provide the collaborating
organization’s Unique Entity Identifier (UEI) number and name. Any other organizations
involved in the component (including the applicant organization) are included in
subaward/consortium budget forms.
From an administrative perspective, the entire component (minus any work done by the
applicant organization) is treated as a subaward/consortium to the applicant organization.
The structure of the application reflects where the proposed work is being done, not the flow
of funds. eRA systems use the Unique Entity Identifier (UEI) numbers included on budget
forms to determine the flow of funds.
B. Collaborating Organization as a Consortium in a Component:
When a collaborating organization does not have a leadership role for a component, then the
applicant organization is the component lead, and any collaborating organizations are
included using the subaward/consortium budget form.
M - 14

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.130 - Program Overview
Multi-project Application Component Forms
You must complete a set of forms for each component.
The assembled application image created for a multi-project application has a predefined order.
For information on multi-project application assembly, see the How eRA Assembles Multi-project
Applications file.
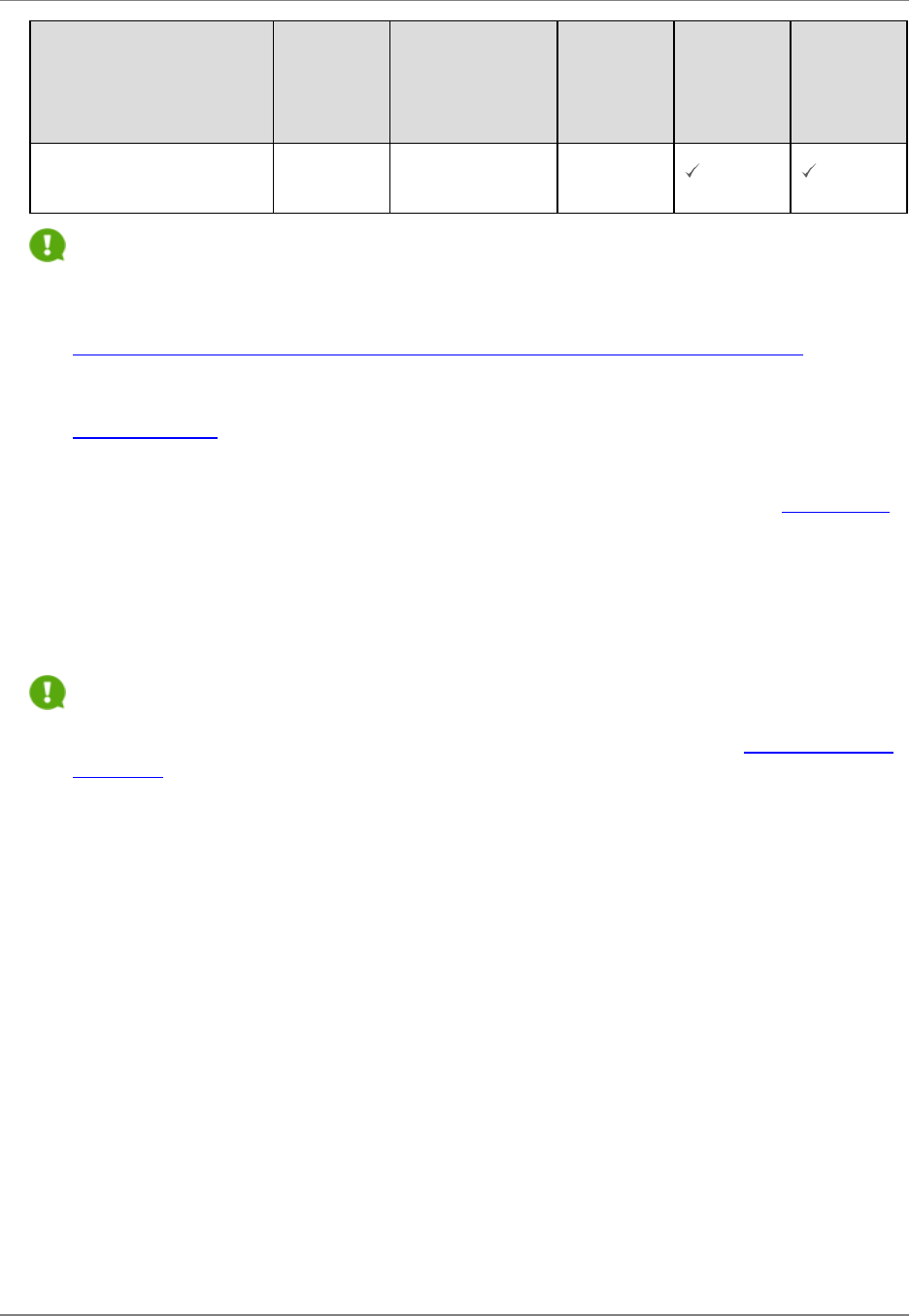
The chart below summarizes which forms must be completed for each component.
Component Data Forms
Form Overall
Admin Core,
Core Project,
Other named
components
Indiv
Career
Dev
Career
Dev
NRSA
Training
SF424 R&R cover
PHS 398 Cover Page
Supplement
R&ROther Project
Information
Project/Performance Sites
R&R Sr/Key Person Profile
(Expanded)
PHS Human Subjects and
Clinical Trials Information
PHS Assignment Request
Form
Optional
R&R Budget
R&R Subaward Budget
Attachment
Optional Optional Optional
PHS 398 Training Budget
Training Subaward
Budget Attachment Form
Optional
PHS Additional Indirect
Costs
Optional
PHS 398 Research Plan
PHS 398 Career
Development Award
Supplemental Form
M - 15
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.130 - Program Overview
Form Overall
Admin Core,
Core Project,
Other named
components
Indiv
Career
Dev
Career
Dev
NRSA
Training
PHS 398 Research
Training Program Plan
Disclosure Requirements Regarding Ties to Foreign Countries
Effective for competing applications submitted on or after September 5, 2023, applicants will be
required to disclose all funded and unfunded relationships with foreign countries, using the
Required Disclosures of Foreign Affiliations or Relationships to Foreign Countries form, (referred to
hereafter as the SBIR STTR Foreign Disclosure Form) for all owners and covered individuals.
Upon request, applicants will submit the completed SBIR STTR Foreign Disclosure Form via the
Just-In-Time (JIT) process described in the NIH GPS section 2.5.1 Just-in-Time Procedures. The
SBIR STTR Foreign Disclosure Form and any additional agency-specific information must be
submitted electronically using the Just-in-Time feature in the eRA Commons. Applicants must
continue to comply with NIH Other Support disclosure requirements as provided in Section 2.5.1.
SBC applicants applying to CDC and FDA will follow each agency’s policies for submitting
additional documents during the pre-award process. Applicants may be required to provide
similar information on the SBIR STTR Foreign Disclosure Form that is also submitted as a part of
the other support reporting for senior/key personnel identified in the application. Applicants that
do not submit the completed SBIR STTR Foreign Disclosure Form during the JIT process will not be
considered for funding.
Denial of Awards
Applicants are encouraged to consider whether their entity’s relationships with foreign countries
of concern will pose a security risk. Prior to issuing an award, NIH, CDC, and FDA will determine
whether the SBC submitting the application:
l
has an owner or covered individual that is party to a malign foreign talent recruitment
program;
l
has a business entity, parent company, or subsidiary located in the People’s Republic of China
or another foreign country of concern; or
l
has an owner or covered individual that has a foreign affiliation with a research institution
located in the People’s Republic of China or another foreign country of concern.
A finding of foreign involvement with countries of concern will not necessarily disqualify an
applicant. NIH, CDC, and FDA will provide SBC applicants the opportunity to address any identified
security risks prior to award. Final award determinations will be based on whether the applicant’s
involvement falls within any of the following risk criteria, per the Act:
• interfere with the capacity for activities supported by NIH, CDC, or FDA to be carried out;
• create duplication with activities supported by NIH, CDC, or FDA;
• present concerns about conflicts of interest;
• were not appropriately disclosed to NIH, CDC, or FDA;
• violate Federal law or terms and conditions of NIH, CDC, or FDA; or
M - 16

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.130 - Program Overview
• pose a risk to national security.
NIH, CDC, and FDA will not issue an award under the SBIR/STTR program if the covered
relationship with a foreign country of concern identified in this guidance is determined to fall
under any of the criteria provided above, and the risk cannot be resolved.
M - 17

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
M.200 - SF 424 (R&R) Form
The SF 424 (R&R) Form is used in all grant applications.
This form collects information including type of
submission, applicant information, type of applicant, and
proposed project dates.
View larger image
Quick Links
1. Type of Submission
2. Date Submitted and Applicant Identifier
3. Date Received by State and State Application Identifier
4a. Federal Identifier
4b. Agency Routing Identifier
4c. Previous Grants.gov Tracking ID
5. Applicant Information
6. Employer Identification
7. Type of Applicant
8. Type of Application
9. Name of Federal Agency
10. Catalog of Federal Domestic Assistance Number and Title
11. Descriptive Title of Applicant's Project
12. Proposed Project
13. Congressional District of Applicant
14. Project Director/Principal Investigator Contact Information
15. Estimated Project Funding
16. Is Application Subject to Review by State Executive Order 12372 Process?
17. Certification
18. SFLLL (Disclosure of Lobbying Activities) or Other Explanatory Documentation
19. Authorized Representative
20. Pre-application
21. Cover Letter Attachment
Additional Instructions for Multi-project:
Overall Component: Fill in all the SF424 (R&R) Form fields, as they are all collected.
M - 18

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Other Components: You need to fill in only a subset of fields in the SF424 (R&R)
Form. Skip the other fields, as any information provided in them will be discarded.
The fields you must fill in are:
5. Applicant Information
7. Type of Applicant (Optional)
11. Descriptive Title of Applicant's Project
12. Proposed Project
1. Type of Submission
This field is required. Check one of the "Type of Submission" boxes:
Pre-application:
The pre-application option is not used by NIH or other PHS agencies unless specifically noted in a
funding opportunity announcement (FOA).
Application:
An "Application" is a request for financial support of a project or activity submitted on specified
forms and in accordance with NIH instructions. (See NIH Types of Applications for an explanation
of the types of applications).
Changed/Corrected Application:
Check this box if you are correcting either system validation errors or application assembly
problems that occurred during the submission process. Changed/corrected applications must be
submitted before the application due date.
When you submit a changed/corrected application, follow these guidelines:
l
After submission of an application, there is a two-day application viewing window. Prior to the
due date, you may submit a changed/corrected application. Submitting a changed/corrected
application will replace the previous submission and remove the previous submission from
consideration.
l
If you check the “Changed/Corrected Application” box, then "Field 4.c Previous Grants.gov
Tracking ID” is required.
l
Do not use the “Changed/Corrected Application” box to denote a resubmission application.
Resubmission applications will be indicated in “Field 8. Type of Application.” See NIH Glossary
for the definition of Resubmission.
2. Date Submitted and Applicant Identifier
The “Date Submitted” field will auto-populate upon application submission.
Fill in the “Applicant Identifier” field, if applicable. The Applicant Identifier is reserved for applicant
use, not the federal agency to which the application is being submitted.
3. Date Received by State and State Application Identifier
Skip the “Date Received by State” and “State Application Identifier” fields.
M - 19

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
4.a. Federal Identifier
New Applications without Pre-application: Leave this field blank.
New Applications following Pre-application: Enter the agency-assigned pre-application
number.
Resubmission, Renewal, and Revision Applications: The Federal Identifier is required. Include
only the IC and serial number of the previously assigned application / award number (e.g., use
CA987654 from 1R01CA987654-01A1).
4.b. Agency Routing Identifier
Skip the “Agency Routing Identifier” field unless otherwise specified in the FOA or notice in the
NIH Guide for Grants & Contracts.
Applications in response to a NIH Notice of Special Interest require the notice number (e.g., NOT-
IC-FY-XXX) to be entered into this field in order to assign and track applications and awards for the
described initiative.
4.c. Previous Grants.gov Tracking ID
The “Previous Grants.gov Tracking ID” field is required if you checked the “Changed/Corrected
Application” box in “Field 1. Type of Submission.” A Tracking ID number is of the form, for
example, GRANT12345678.
5. Applicant Information
The "Applicant Information" fields reflect information for the applicant organization, not a specific
individual.
Additional Instructions for Multi-project:
Other Components: The “Applicant Information” section is required and applies to
the lead organization of the component.
Unique Entity Identifier (UEI):
This field is required.
Enter the UEI of the applicant organization.
This UEI must match the number entered in the eRA Commons Institutional Profile (IPF) for the
applicant organization. The applicant’s Authorized Organization Representative (AOR) is
encouraged to confirm that a UEI has been entered into the eRA Commons IPF prior to application
submission. The same UEI should be used in the eRA Commons IPF, Grants.gov, System for Award
Management (SAM) registration, and in the UEI field in the application.
If your organization does not already have a UEI, you will need to go to the System for Award
Management (SAM.gov) to register and obtain a UEI.
M - 20
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Additional Instructions for Multi-project:
Other Components: If a component is led by an organization other than the
applicant organization, then you must provide the lead organization’s4UEI. If the
organization does not already have a UEI, you will need to go to the System for
Award Management (SAM.gov) to obtain a UEI. However, the lead organization does
not need to be registered in SAM or in eRA Commons at the time of application.
SAM registration is encouraged since it helps staff process your application if you
are selected for funding.
Legal Name:
Enter the legal name of the organization.
Department:
Enter the name of the primary organizational department, service, laboratory, or equivalent level
within the organization.
Division:
Enter the name of the primary organizational division, office, major subdivision, or equivalent level
within the organization.
Street1:
This field is required. Enter the first line of the street address for the applicant organization.
Street2:
Enter the second line of the street address for the applicant organization.
City:
This field is required. Enter the city for the address of the applicant organization.
County/Parish:
Enter the county/parish for the address of the applicant organization.
State:
This field is required if the applicant organization is located in the United States or its territories.
Enter the state or territory where the applicant organization is located.
Province:
If “Country” is Canada, enter the province of the applicant organization; otherwise, skip the
“Province” field.
Country:
This field is required. Select the country for the address of the applicant organization.
ZIP/Postal Code:
The ZIP+4 is required if the applicant organization is located in the United States. Otherwise, the
postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the applicant
organization.
M - 21

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Person to be contacted on matters involving this application
This information is for the administrative contact (e.g., AOR or business official), not the PD/PI. This
person is the individual to be notified if additional information is needed and/or if an award is
made.
Prefix:
Enter or select the prefix, if applicable, for the name of the person to contact on matters related to
this application.
First Name:
This field is required. Enter the first (given) name of the person to contact on matters related to this
application.
Middle Name:
Enter the middle name of the person to contact on matters related to this application.
Last Name:
This field is required. Enter the last (family) name of the person to contact on matters related to
this application.
Suffix:
Enter or select the suffix, if applicable, for the name of the person to contact on matters related to
this application.
Position/Title:
Enter the position/title for the person to contact on matters related to this application.
Street1:
This field is required. Enter the first line of the street address for the person to contact on matters
related to this application.
Street2:
Enter the second line of the street address for the person to contact on matters related to this
application.
City:
This field is required. Enter the city for the address of the person to contact on matters related to
this application.
County/Parish:
Enter the county/parish for the address of the person to contact on matters related to this
application.
State:
This field is required if the person to contact on matters related to this application is located in the
United States or its Territories. Enter the state or territory where the person to contact on matters
related to this application is located.
M - 22

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Province:
If “Country” is Canada, enter the province for the person to contact on matters related to this
application; otherwise, skip the “Province” field.
Country:
Select the country for the address of the person to contact on matters related to this application.
ZIP/Postal Code:
The ZIP+4 is required if the person to contact on matters related to this application is in the United
States. Otherwise, the postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal
code of the person to contact on matters related to this application.
Phone Number:
This field is required. Enter the daytime phone number for the person to contact on matters
related to this application.
Fax Number:
Enter the fax number for the person to contact on matters related to this application.
E-mail:
Enter the e-mail address for the person to contact on matters related to this application. Only one
e-mail address is allowed, but it may be a distribution list.
6. Employer Identification
This field is required.
Enter either the organization’s Taxpayer Identification Number (TIN) or Employer Identification
Number (EIN) as assigned by the Internal Revenue Service. If your organization is not in the United
States, enter 44-4444444. Your EIN may be 12 digits (e.g., Payment Management System (PMS)
Entity Identification Number), and if this is the case, enter all 12 digits.
7. Type of Applicant
This field is required.
In the first field under “7. Type of Applicant,” enter the appropriate applicant type. If your applicant
type is not specified (e.g., for eligible Agencies of the Federal Government), select “X: Other
(specify),” and indicate the name (e.g., the appropriate federal agency) in the space below.
Additional Instructions for Multi-project:
Other Components: You may fill out “7. Type of Applicant,” but it is optional.
Other (Specify):
Complete only if “X. Other (specify)” is selected as the "Type of Applicant."
Women Owned:
Do not use the "Women Owned" checkbox.
Socially and Economically Disadvantaged:
Do not use the "Socially and Economically Disadvantaged" checkbox.
M - 23

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Note: NIH, CDC, and FDA use the Business Type information provided in the System for Award
Management entity record for the applicant organization, rather than the “Woman Owned” and
“Socially and Economically Disadvantaged” checkboxes, to determine the small business
organization type. For more information, see the NIH Guide Notice on Small Business Organization
Type Information Pulled from System for Award Management Record Rather than Grant
Application Form.
8. Type of Application
This field is required.
Select the type of application. Check only one application type. Use the following list of existing
definitions to determine what application type you have. For more information, see NIH Types of
Applications.
l
New. Check this option when submitting an application for the first time or in accordance
with other submission policies. See the NIH Grants Policy Statement, Section 2.3.7.4:
Submission of Resubmission Application.
l
Resubmission. Check this option when submitting a revised (altered or corrected) or
amended application. See also the NIH Application Submission Policies. If your
application is both a “New/Revision/Renewal” and a “Resubmission,” check only the
“Resubmission” box.
l
Renewal. Check this option if you are requesting additional funding for a period
subsequent to that provided by a current award. A renewal application competes with all
other applications and must be developed as fully as if the applicant were applying for the
first time.
l
Continuation. The box for “Continuation” is used only for specific FOAs.
l
Revision. Check this option for competing revisions and non-competing administrative
supplements. For more information on competing revisions, see NIH Competing
Revisions. For more information on administrative supplements, see NIH Administrative
Supplements.
If Revision, mark appropriate box(es).
You may select more than one.
A. Increase Award
B. Decrease Award
C. Increase Duration
D. Decrease Duration
E. Other (specify)
If “E. Other (specify)” is selected, specify in the space provided.
The boxes for options B, C, D, and E will generally not be used and should not be selected unless
specifically addressed in a particular FOA.
Is this application being submitted to other agencies? What Other Agencies?
In the field “Is this application being submitted to other agencies?” check “Yes” if one or more of
the specific aims submitted in your application is also contained in a similar, identical, or essentially
M - 24

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
identical application submitted to another federal agency.
Otherwise, check "No."
If you checked "Yes," indicate the agency or agencies to which the application has been submitted.
9. Name of Federal Agency
The “Name of Federal Agency” field is pre-populated from the opportunity package and reflects
the agency from which assistance is being requested with this application.
10. Catalog of Federal Domestic Assistance Number and Title
This field is pre-populated from the opportunity package and reflects the Catalog of Federal
Domestic Assistance (CFDA) number of the program under which assistance is requested.
This field may be blank if you are applying to an opportunity that references multiple CFDA
numbers. When this field is blank, leave it blank. The appropriate CFDA number will be
automatically assigned by the agency once the application is assigned to the appropriate
awarding component.
11. Descriptive Title of Applicant’s Project
This field is required.
Additional Instructions for Multi-project:
Other Components: The “Descriptive Title of Applicant’s Project” section is
required.
Enter a brief descriptive title of the project.
The descriptive title is limited to 200 characters, including spaces and punctuation.
New Applications: You must have a title different than any other NIH or other PHS Agency
project submitted for the same application due date with the same Project Director/Principal
Investigator (PD/PI).
Resubmission or Renewal Applications: You should normally have the same title as the previous
grant or application; however, if the specific aims of the project have significantly changed, choose
a new title.
Revision Applications: You must have the same title as the currently funded grant.
12. Proposed Project
Additional Instructions for Multi-project:
Other Components: The “Proposed Project” section is required.
Start Date:
This field is required. Enter the proposed start date of the project. The start date is an estimate, and
is typically at least nine months after application submission. The project period should not exceed
M - 25

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
what is allowed in the FOA.
Ending Date:
This field is required. Enter the proposed ending date of the project.
13. Congressional District of Applicant
Enter the Congressional District as follows: a 2-character state abbreviation, a hyphen, and a 3-
character district number. Examples: CA-005 for California’s 5th district, VA-008 for Virginia’s 8th
district.
If outside the United States, enter 00-000.
For States and U.S. Territories with only a single congressional district, enter “001” for the district
number.
For jurisdictions with no representative, enter “099.”
For jurisdictions with a nonvoting delegate, enter “098” for the district number. Example: DC-098
or PR-098.
If you do not know your Congressional District: Go to The United States House of
Representatives website and search for your Congressional District by entering your ZIP+4. If you
do not know your ZIP+4, look it up on the USPS Look Up Zip Code website.
14. Project Director/Principal Investigator Contact Information
This information is for the PD/PI. The PD/PI is the individual responsible for the overall scientific
and technical direction of the project.
In the eRA Commons profile, the person listed here in “14. Project Director/Principal Investigator
Contact Information” must be affiliated with the applicant organization entered in “5. Applicant
Information.” If you are proposing research at an institute other than the one you are currently at,
do not create a separate Commons account with the proposed applicant organization. For
additional information on creating affiliations for users in the eRA Commons, see eRA Account
Management System's Online Help.
If submitting an application reflecting multiple PD/PIs, the individual listed here as the Contact
PD/PI in “14. Project Director/Principal Investigator Contact Information” will be the first PD/PI
listed in M.240 - R&RSenior/Key Person Profile (Expanded) Form.
See M.240 - R&R Senior/Key Person Profile (Expanded) Form for additional instructions for
multiple PD/PIs. To avoid potential errors and delays in processing, ensure that the information
provided in this section is identical to the PD/PI profile information contained in the eRA
Commons.
Prefix:
Enter or select the prefix, if applicable, for the name of the PD/PI.
First Name:
This field is required. Enter the first (given) name of the PD/PI.
Middle Name:
Enter the middle name of the PD/PI.
M - 26

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Last Name:
This field is required. Enter the last (family) name of the PD/PI.
Suffix:
Enter or select the suffix, if applicable, for the PD/PI. Do not use this field to record degrees (e.g.,
Ph.D. or M.D.). Degrees for the PD/PI are requested separately in the R&R Senior/Key Person
Profile (Expanded) Form.
Position/Title:
Enter the position/title of the PD/PI.
Organization Name:
This field is required. This field may be pre-populated from the applicant information section in
this form.
Department:
Enter the name of primary organizational department, service, laboratory, or equivalent level
within the organization of the PD/PI.
Division:
Enter the name of primary organizational division, office, major subdivision, or equivalent level
within the organization of the PD/PI.
Street1:
This field is required. Enter first line of the street address for the PD/PI.
Street2:
Enter the second line of the street address for the PD/PI.
City:
This field is required. Enter the city for the address of the PD/PI.
County/Parish:
Enter the county/parish for the address of the PD/PI.
State:
This field is required if the PD/PI is located in the United States or its Territories. Enter the state or
territory where the PD/PI is located.
Province:
If “Country” is Canada, enter the province for the PD/PI; otherwise, skip the “Province” field.
Country:
Select the country for the PD/PI.
ZIP/Postal Code:
The ZIP+4 is required if the PD/PI address is in the United States. Otherwise, the postal code is
optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the PD/PI.
Phone Number:
This field is required. Enter the daytime phone number for the PD/PI.
M - 27

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Fax Number:
Enter the fax number for the PD/PI.
E-mail:
This field is required. Enter the e-mail address for the PD/PI.
15. Estimated Project Funding
All four fields in “15. Estimated Project Funding” are required.
a. Total Federal Funds Requested
Enter the total federal funds, including Direct Costs and F&A Costs (Indirect Costs), requested for
the entire project period.
b. Total Non-Federal Funds
For applications to NIH and other PHS agencies, enter “0” in this field unless cost sharing is a
requirement for the specific FOA.
c. Total Federal & Non-Federal Funds
Enter the total federal and non-federal Funds requested. The amount in this field will be the same
as the amount in the “Total Federal Funds Requested” field unless the specific FOA indicates that
cost sharing is a requirement.
d. Estimated Program Income
Indicate any program income estimated for this project, if applicable.
16. Is Application Subject to Review by State Executive Order 12372 Process?
Applicants should check “No, Program is not covered by E.O. 12372.”
17. Certification
This field is required.
The list of NIH and other PHS agencies Certifications, Assurances, and other Policies is found in the
NIH Grants Policy Statement, Section 4: Public Policy Requirements and Objectives.
The applicant organization is responsible for verifying its eligibility and the accuracy, validity, and
conformity with the most current institutional guidelines of all the administrative, fiscal, and
scientific information in the application, including the Facilities and Administrative rate. Deliberate
withholding, falsification, or misrepresentation of information could result in administrative
actions, such as withdrawal of an application, suspension and/or termination of an award,
debarment of individuals, as well as possible criminal and/or civil penalties. The signer further
certifies that the applicant organization will be accountable both for the appropriate use of any
funds awarded and for the performance of the grant-supported project or activities resulting from
this application. The grantee institution may be liable for the reimbursement of funds associated
with any inappropriate or fraudulent conduct of the project activity.
Check “I agree” to provide the required certifications and assurances.
M - 28

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
18. SFLLL (Disclosure of Lobbying Activities) or Other Explanatory
Documentation
If applicable, attach the SFLLL or other explanatory document as per FOA instructions.
If unable to certify compliance with the Certification in the “17. Certification” section above, attach
an explanation. Additionally, as applicable, attach the SFLLL (Standard Form LLL, Disclosure of
Lobbying Activities) or other documents in this item.
For more information:
See the NIH Grants Policy Statement, Section 4.1.17: Lobbying Prohibition, and the NIH Lobbying
Guidance for Grantee Activities page.
19. Authorized Representative
The authorized representative is equivalent to the individual with the organizational authority to
sign for an application. This individual is otherwise known as the authorized organization
representative (AOR) in Grants.gov or the signing official (SO) in eRA Commons.
Prefix:
Enter or select the prefix, if applicable, for the name of the AOR/SO.
First Name:
This field is required. Enter the first (given) name of the AOR/SO
Middle Name:
Enter the middle name of the AOR/SO.
Last Name:
This field is required. Enter the last (family) name of the AOR/SO.
Suffix:
Enter or select the suffix, if applicable, for the AOR/SO.
Position/Title:
This field is required. Enter the position/title of the name of the AOR/SO.
Organization Name:
This field is required. Enter the name of the organization for the AOR/SO.
Department:
Enter the name of the primary organizational department, service, laboratory, or equivalent level
within the organization for the AOR/SO.
Division:
Enter the name of the primary organizational division, office, major subdivision, or equivalent level
within the organization for the AOR/SO.
Street1:
This field is required. Enter the first line of the street address for the AOR/SO.
M - 29

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Street2:
Enter the second line of the street address for the AOR/SO.
City:
This field is required. Enter the city for the address of the AOR/SO.
County/Parish:
Enter the county/parish for the address of the AOR/SO.
State:
This field is required if the AOR/SO is located in the United States or its Territories. Enter the state
or territory where the AOR/SO is located.
Province:
If “Country” is Canada, enter the province for the AOR/SO; otherwise, skip the “Province” field.
Country:
Select the country for the address of the AOR/SO.
ZIP/Postal Code:
The ZIP+4 is required if the AOR/SO is in the United States. Otherwise, the postal code is optional
Enter the ZIP+4 (nine-digit postal code) or postal code of the AOR/SO.
Phone Number:
This field is required. Enter the daytime phone number for the AOR/SO.
Fax Number:
Enter the fax number for the AOR/SO.
Email:
This field is required. Enter the e-mail address for the AOR/SO.
Signature of Authorized Representative:
Grants.gov will record the electronic signature for the AOR/SO who submits the application.
It is the organization’s responsibility to assure that only properly authorized individuals sign in this
capacity and/or submit the application to Grants.gov.
Date Signed:
Grants.gov will generate this date upon application submission.
20. Pre-application
Unless specifically noted in a FOA, NIH and other PHS agencies do not use pre-applications. The
"Pre-application" attachment field should not be used for any other purpose.
If permitted by your FOA, attach this information as a PDF.
21. Cover Letter Attachment
The cover letter is for internal use only and will not be shared with peer reviewers.
M - 30

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
Who must complete the “Cover Letter Attachment”:
Refer to the “content” list below for items that are permitted, as well as for specific situations in
which a cover letter must be included.
A cover letter must not be included with post-award submissions, such as administrative
supplements, change of grantee institution, or successor-in-interest.
Format:
Attach the cover letter, addressed to the Division of Receipt and Referral, in accordance with the
FOA and/or these instructions.
Attach the cover letter in the correct location, specifically verifying that the cover letter has not
been uploaded to the “20. Pre-application” field which is directly above the “21. Cover
Letter Attachment” field. This will ensure the cover letter attachment is kept separate from the
assembled application in the eRA Commons and made available only to appropriate staff.
Content:
Do not use the cover letter to communicate application assignment preferences. The Assignment
Request Form is provided for that purpose.
The letter should contain any of the following information, as applicable:
1. Application title.
2. Title of FOA (PA or RFA).
3. For late applications (see Late Application policy on NIH's Application Submission
Policies) include specific information about the timing and nature of the delay.
4. For changed/corrected applications submitted after the due date, a cover letter is
required, and it must explain the reason for late submission of the changed/corrected
applications. If you already submitted a cover letter with a previous submission and are
now submitting a late change/corrected application, you must include all previous cover
letter text in the revised cover letter attachment. The system does not retain any
previously submitted cover letters; therefore, you must repeat all information previously
submitted in the cover letter as well as any additional information.
5. Explanation of any subaward budget components that are not active for all budget
periods of the proposed grant (see M.310 – R&R Subaward Budget Attachment(s) Form).
6. Statement that you have attached any required agency approval documentation for the
type of application submitted. This may include approval for applications that request
$500,000 or more, approval for Conference Grant or Cooperative Agreement (R13 or U13),
etc. It is recommended that you include the official communication from an NIH official as
part of your cover letter attachment.
7. When intending to submit a video as part of the application, the cover letter must include
information about the intent to submit it; if this is not done, the video will not be
accepted. See NIH Grants Policy Statement, Section 2.3.7.7: Post Submission Grant
Application Materials for additional information.
8. Include a statement in the cover letter if the proposed studies will generate large-scale
human or non-human genomic data as detailed in the NIH Genomic Data Sharing Policy
(see the NIH Grants Policy Statement, Section 2.3.7.10: NIH Genomic Data Sharing and
Section 8.2.3.3: Genomic Data Sharing (GDS) Policy/Policy for Genome-Wide Association
Studies (GWAS)).
M - 31

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.200 - SF 424 (R&R) Form
9. Include a statement in the cover letter if the proposed studies involve human fetal tissue
obtained from elective abortions (HFT), regardless of whether or not Human Subjects are
involved and/or there are costs associated with the HFT. For further information on
HFTpolicy refer to the NIHGrants Policy Statement, Section 2.3.7.11 Human Fetal Tissue
from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research and Section 4.1.14.2
Human Fetal Tissue from Elective Abortions.
M - 32

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.210 - PHS 398 Cover Page Supplement Form
M.210 - PHS 398 Cover Page
Supplement Form
The PHS 398 Cover Page Supplement Form is used for all
grant applications except fellowships. This form collects
information on human subjects, vertebrate animals,
program income, human embryonic stem cells, inventions
and patents, and changes of investigator/change of
institution.
View larger image
Quick Links
1. Vertebrate Animals Section
2. Program Income Section
3. Human Embryonic Stem Cell Section
4. Human Fetal Tissue Section.
5. Inventions and Patents Section (for Renewal applications)
6. Change of Investigator/Change of Institution Section
1. Vertebrate Animals Section
Are vertebrate animals euthanized?
You must answer this question if you answered “Yes” to the question “Are Vertebrate Animals
Used?” on the M.220 – R&R Other Project Information Form.
Check "Yes" or "No" to indicate whether vertebrate animals in the project are euthanized.
Additional Instructions for Multi-project:
Overall Component: If vertebrate animals will be euthanized in any Component,
then you must answer “Yes” to the “Are vertebrate animals euthanized?” question.
If “Yes” to euthanasia: Is method consistent with American Veterinary Medical Association
(AVMA) guidelines?
You must answer this question if you answered “Yes” to the “Are vertebrate animals euthanized?”
question above. Check “Yes” or “No” to indicate whether the method of euthanasia is consistent
with the AVMA Guidelines for the Euthanasia of Animals.
For more information: See AVMA Guidelines for the Euthanasia of Animals.
M - 33

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.210 - PHS 398 Cover Page Supplement Form
If “No” to AVMA guidelines, describe method and provide scientific justification:
If you answered “No” to the “Is method consistent with AVMA guidelines?” question, you must
describe (in 1000 characters or fewer) the method of euthanasia and provide a scientific
justification for its use. This justification will be reviewed by Office of Laboratory Animal Welfare
(OLAW).
If you answered “Yes” to the “Is method consistent with AVMA guidelines” question, skip this
question.
2. Program Income Section
Is program income anticipated during the periods for which the grant support is requested?
This field is required.
If program income is anticipated during the periods for which grant support is requested, check
“Yes,” and complete the rest of the “Program Income” section.
If no program income is anticipated, check “No” and skip the rest of the “Program Income” section.
Additional Instructions for Multi-project:
Overall Component: If you anticipate program income on any component, then
answer "Yes." Skip the other fields, as any information provided in them will be
discarded. Instead of program income information being provided in the Overall
Component, a system-generated summary of all program income information that
you provide in Other Components will be included in the summaries section of the
assembled application image.
Other Component: If you anticipate program income on any component, then
answer "Yes." Provide the budget period, anticipated amount, and source
information.
Budget Period:
Enter the budget periods for which program income is anticipated. If the application is funded, the
Notice of Grant Award will provide specific instructions regarding the use of such income.
Anticipated Amount ($):
Enter the amount of anticipated program income for each budget period listed.
Source(s):
Enter the source of anticipated program income for each budget period listed.
3. Human Embryonic Stem Cells Section
Use the following instructions to complete the fields in this section.
For additional guidance, see the NIH Grants Policy Statement, Section 4.1.13: Human Stem Cell
Research.
Does the proposed project involve human embryonic stem cells?
This field is required.
M - 34

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.210 - PHS 398 Cover Page Supplement Form
If the proposed project involves human embryonic stem cells (hESC), check “Yes” and complete the
rest of the “Human Embryonic Stem Cells” section.
l
Use of the cell lines must be in accordance with the NIH Guidelines for Human Stem Cell
Research.
If the proposed project does not involve hESC, check “No” and skip the rest of the “Human
Embryonic Stem Cells” section.
Additional Instructions for Multi-project:
Overall Component: If human embryonic stem cells are used in any Component,
then you must answer “Yes.”
Specific stem cell line cannot be referenced at this time. One from the registry will be used.
If you will use hESC but a specific line from the NIH hESC Registry cannot be chosen at the time of
application submission, check this box.
If you cannot specify which cell lines will be used at the time of application submission, specific cell
line information will be required as Just-in-Time information prior to award.
Additional Instructions for Multi-project:
Overall and Other Components: If you cannot choose an appropriate cell line from
the registry at this time, provide a justification in the M.400 - PHS 398 Research Plan
Form, Research Strategy attachment.
Cell Line(s):
List the 4-digit registration number of the specific cell line(s) from the NIH hESC Registry (e.g.
0123). Up to 200 lines can be added.
Additional Instructions for Multi-project:
Overall Component: Skip the "Cell Line(s)" field, as any information provided here
will be discarded. Instead of cell line information being provided in the Overall
Component, a system-generated summary of all cell line information that you
provide in Other Components will be included in the summaries section of the
assembled application image.
Other Component: Provide any cell line information relevant to the work being
done in that component.
For more information:
See NIH’s Stem Cell Information page for additional information on stem cells, Federal policy
statements, and guidelines on federally funded stem cell research.
M - 35
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.210 - PHS 398 Cover Page Supplement Form
4. Human Fetal Tissue Section
Does the proposed project involve human fetal tissue from elective abortions?
This field is required.
If the proposed project involves the use of human fetal tissue obtained from elective abortions
(HFT), check “Yes” and complete the rest of the “Human Fetal Tissue” section.
If the proposed project does not involve the use of human fetal tissue obtained from elective
abortions (HFT), check “No” and skip the rest of the “Human Fetal Tissue” section.
Additional Instructions for Multi-project:
If the answer is "yes" then provide the HFT Compliance Assurance:
If the proposed project involves the use of human fetal tissue obtained from elective abortions
(HFT), the applicant must provide a letter, signed by the PD/PI, assuring the HFT donating
organization or clinic adheres to the requirements of the informed consent process and
documenting that HFT was not obtained or acquired for valuable consideration. The PDF-
formatted letter must be named ‘HFTComplianceAssurance.pdf’.
If the answer is “yes” then provide the HFT Sample IRB Consent Form
If the proposed project involves the use of human fetal tissue obtained from elective abortions
(HFT), provide a blank sample of the IRB-approved consent form. The PDF-formatted form must be
a blank sample and named ‘HFTSampleIRBConsentForm.pdf’.
o The informed consent for use of HFT from elective abortions requires language that
acknowledges informed consent for donation of HFT was obtained by someone other than the
person who obtained the informed consent for abortion, that informed consent for donation of
HFT occurred after the informed consent for abortion was obtained will not affect the method of
abortion, and that no enticements, benefits, or financial incentives were used at any level of the
process to incentivize abortion or the donation of HFT. The form must be signed by both the
woman and the person who obtains the informed consent.
For further information on HFTpolicy refer to the NIHGrants Policy Statement, Section 2.3.7.11
Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research and
Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
5. Inventions and Patents Section (for Renewal applications)
Who must complete the “Invention and Patents” section:
Complete the "Inventions and Patents" section only if you are submitting a renewal application or a
resubmission of a renewal application.
Inventions and Patents:
If no inventions were conceived or reduced to practice during the course of work under this
project, check “No” and skip the remainder of the “Inventions and Patents” section.
If any inventions were conceived or first actually reduced to practice during the previous period of
support, check “Yes.”
M - 36

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.210 - PHS 398 Cover Page Supplement Form
NIH recipient organizations must promptly report inventions to the Division of Extramural
Inventions and Technology Resources (DEITR) Branch of the Office of Policy for Extramural
Research Administration (OPERA), OER, NIH, 6705 Rockledge Drive, Bethesda, MD 20892-2750,
(301) 435-1986. You must report inventions in compliance with regulations at 37 CFR 401.14,
which are described at Interagency Edison (iEdison). The grantee is required to submit reports
electronically using iEdison. See the NIH Grants Policy Statement, Section 8.4.1.6: Invention
Reporting.
Previously Reported:
If you answered “Yes” to the “Inventions and Patents” question, indicate whether this information
has been reported previously to the NIH or PHS agency or to the applicant organization official
responsible for patent matters.
6. Change of Investigator/Change of Institution Section
Change of Project Director/Principal Investigator:
Check this box if your application reflects a change in project director/principal investigator
(PD/PI) from that indicated on your previous application or award. Note that this box not
applicable to a new application, nor is a change in PD/PI permitted for revision applications.
For a multiple PD/PI application, check this box if this application represents a change in the
contact PI.
If you check the box, fill in the rest of the “Change of PD/PI” section with the information for the
former PD/PI according to the instructions below.
Prefix:
Enter or select the prefix, if applicable, for the former PD/PI.
First Name:
Enter the first (given) name of the former PD/PI.
Middle Name:
Enter the middle name of the former PD/PI.
Last Name:
Enter the last (family) name of the former PD/PI.
Suffix:
Enter or select the suffix, if applicable, for the former PD/PI.
Change of Grantee Institution:
Check this box if your application reflects a change in grantee institution from that indicated
on your previous application or award. This question is not applicable to new applications.
Name of Former Institution:
Enter the name of the former institution if this application reflects a change in grantee
institution.
M - 37

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
M.220 - R&R Other Project Information
Form
The R&R Other Project Information Form is used for all
grant applications. This form includes questions on the
use of human subjects, vertebrate animals, and
environmental impact. This form also has fields to upload
an abstract, project narrative, references, information on
facilities, and equipment lists.
View larger image
Quick Links
1. Are Human Subjects Involved?
1a. If YES to Human Subjects
2. Are Vertebrate Animals Used?
2a. If YES to Vertebrate Animals
3. Is proprietary/privileged information included in the application?
4. Environmental Questions
5. Is the research performance site designated, or eligible to be designated, as a historic place?
6. Does this project involve activities outside of the United States or partnerships with inter-
national collaborators?
7. Project Summary/Abstract
8. Project Narrative
9. Bibliography & References Cited
10. Facilities & Other Resources
11. Equipment
12. Other Attachments
1. Are Human Subjects Involved?
This field is required.
If activities involving human subjects are planned at any time during the proposed project at any
performance site, check “Yes.” Check “Yes” even if the proposed project is exempt from regulations
for the Protection of Human Subjects, or if activities involving human subjects are anticipated
within the period of award but plans are indefinite, or if the proposed activities include public
health surveillance activities described in 45 CFR 46.102(l)(2).
M - 38
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
If activities involving human subjects are not planned at any time during the proposed project at
any performance site, select “No” and skip the rest of the "Are Human Subjects Involved" section.
Whether you answer “Yes” or “No” to the “Are Human Subjects Involved?” question here, your
answer will populate the relevant field in the M.500 – PHS Human Subjects and Clinical Trials
Information form (see exception for Training Applications in the Training-specific instructions).
Follow the M.500 – PHS Human Subjects and Clinical Trials Information form instructions to
complete the relevant questions in that form.
Need help determining whether your application includes human subjects? Check out the
NIH Research Involving Human Subjects website for information, including an Infopath
Questionnaire designed to walk applicants through the decision process.
Note on the use of human specimens or data: Applications involving the use of human
specimens or data may or may not be considered to be research involving human subjects,
depending on the details of the materials to be used. If you check “No” to “Are Human Subjects
Involved?” but your application proposes using human specimens or data, you will be required to
provide a clear justification about why this use does not constitute human subjects research.
Follow the M.500 – PHS Human Subjects and Clinical Trials Information form instructions.
For more information on human biospecimens or data: Refer to the NIH page on Frequently
Asked Questions on Human Specimens, Cell Lines, or Data and the Research Involving Private
Information or Biological Specimens flowchart.
Additional Instructions for Multi-project:
Overall Component: If activities involving human subjects are planned at any time
during the proposed project at any performance site and/or on any Other
Component, check “Yes” to the “Are Human Subjects Involved?” question and
complete the remaining questions as instructed.
Other Components: Answer only the “Are Human Subjects Involved?” and “Is the
Project Exempt from Federal regulations?” questions.
1.a. If YES to Human Subjects
Your answers here in question “1.a. If YES to Human Subjects” will populate the corresponding
fields in the M.500 – PHS Human Subjects and Clinical Trials Information form.
Is the Project Exempt from Federal regulations? Yes/No
If the project is exempt from federal regulations, check “Yes” and check the appropriate exemption
number.
Human subjects research should only be designated as exempt if all of the proposed research
projects in an application meet the criteria for exemption.
If the project is not exempt from federal regulations, check “No.”
For more information, see the NIH’s Exempt Human Subjects Research infographic.
If yes, check appropriate exemption number 1, 2, 3, 4, 5, 6, 7, 8:
If you selected “Yes” to “Is the Project Exempt from Federal Regulations,” select the appropriate
exemption number.
The categories of research that qualify for exemption are defined in the Common Rule for the
Protection of Human Subjects. These regulations can be found at 45 CFR 46.
M - 39

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
Need help determining the appropriate exemption number? Refer to NIH's Research
Involving Human Subjects Frequently Asked Questions.
The Office for Human Research Protections (OHRP) guidance states that appropriate use of
exemptions described in 45 CFR 46 should be determined by an authority independent from the
investigators (for more information, see OHRP's Frequently Asked Questions). Institutions often
designate their Institutional Review Board (IRB) to make this determination. Because NIH does not
require IRB approval at the time of application, the exemptions designated often represent the
opinion of the PD/PI, and the justification provided for the exemption by the PD/PI is evaluated
during peer review. See NIH Grants Policy Statement Section 4.1.15 for more information.
4. Human Fetal Tissue Section
Notes on public health surveillance activities: Projects involving public health surveillance
activities described in 45 CFR 46.102(l)(2) must answer questions in Section 1.a. as if the exclusion
does not apply. In rare circumstances, applicants may request NIH approval for use of the
exclusion in accordance with Just-in-Time procedures.
Additional Instructions for Multi-project:
Overall Component: Check all the exemptions identified in all the Other
Components.
Other Components: If the Overall Component exemption is only E4 (box 4 is
checked) then no other exemption number can be set for any Other Component.
If no, is the IRB review Pending? Yes/No
If IRB review is pending, check “Yes.”
Applicants should check “Yes” to the question “Is the IRB review Pending?” even if the IRB
review/approval process has not started by the time of submission.
If IRB review is not pending (e.g., if the review is complete), check “No.”
Additional Instructions for Multi-project:
Other Components: Skip the "If no, is the IRBreview Pending?" question.
IRB Approval Date:
Enter the latest IRB approval date (if available). Leave blank if IRBapproval is pending.
An IRB approval date is not required at the time of submission when IRB review is pending. This
may be requested later in the pre-award cycle as a Just-In-Time requirement. See the NIH Grants
Policy Statement, Section 2.5.1: Just-in-Time Procedures for more information.
Additional Instructions for Multi-project:
Other Components: Skip the “IRB Approval Date” question.
Human Subject Assurance Number:
Enter the approved Federalwide Assurance (FWA) number that the applicant has on file with
OHRP. Enter the 8-digit number. Do not enter “FWA” before the number.
Enter “None” if the applicant organization does not have an approved FWA on file with OHRP. In
this case, the applicant organization, by the signature in the Certification section on the M.200 -
SF424 (R&R) Form, is declaring that it will comply with 45 CFR 46 and proceed to obtain a FWA
M - 40

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
(see Office for Human Research Protections website). Do not enter the FWA number of any
collaborating institution.
Additional Instructions for Multi-project:
Other Components: Skip the “Human Subject Assurance Number” field.
2. Are Vertebrate Animals Used?
This field is required.
If activities involving vertebrate animals are planned at any time during the proposed project at
any performance site, check “Yes.” Otherwise, check “No” and skip the rest of the “2. Are
Vertebrate Animals Used?” section.
Note that the generation of custom antibodies constitutes an activity involving vertebrate animals.
If animal involvement is anticipated within the period of award but plans are indefinite, check
"Yes."
Additional Instructions for Multi-project:
Overall Component: If activities involving vertebrate animals are planned at any
time during the proposed project at any performance site and/or on any Other
Component, check “Yes” and complete the remaining questions as instructed.
Other Components: Answer only the “Are Vertebrate Animals Used?” question. Skip
the questions in 2.a.
2.a. If YES to Vertebrate Animals
Is the IACUC review Pending?
If an Institutional Animal Care and Use Committee (IACUC) review is pending, check "Yes."
Applicants should check “Yes” to the "Is the IACUC review Pending?" question even if the IACUC
review/approval process has not started by the time of submission.
If IACUC review is not pending (e.g. if the review is complete), check “No."
Additional Instructions for Multi-project:
Overall Component: Complete the “Is the IACUC review Pending?” question when
the answer is “Yes” to “Are Vertebrate Animals Used?”
Other Components: Skip the “Is the IACUC review Pending?” question.
IACUC Approval Date:
Enter the latest IACUC approval date (if available). Leave blank if IACUC approval is pending.
IACUC approval must have been granted within three years of the application submission date to
be valid.
An IACUC approval date is not required at the time of submission. NIH does not require
verification of review and approval of the proposed research by the IACUC before peer review of
the application. However, this information is required under the NIH Grants Policy Statement
Section 2.5.1: Just-in-Time Procedures.
M - 41
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
Additional Instructions for Multi-project:
Other Components: Skip the “IACUC Approval Date” question.
Animal Welfare Assurance Number
Enter the federally approved assurance number, if available.
Enter “None” if the applicant organization does not have an Office of Laboratory Animal Welfare
(OLAW)-approved Animal Welfare Assurance.
To determine whether the applicant organization holds an Animal Welfare Assurance with an
associated number, see the lists of Domestic and Foreign Assured institutions. Do not enter the
Animal Welfare Assurance number for a Project/Performance Site of a collaborating
institution.
When an applicant organization does not have an Animal Welfare Assurance number, the
authorized organization representative’s signature on the application constitutes declaration that
the applicant organization will submit an Animal Welfare Assurance when requested by OLAW.
If the animal work will be conducted at an institution with an Animal Welfare Assurance and the
applicant organization does not have the following:
l
an animal care and use program;
l
facilities to house animals and conduct research on site; and
l
IACUC;
then, the applicant must obtain an Inter-institutional Assurance from OLAW prior to an award.
Additional Instructions for Multi-project:
Other Components: Skip the “Animal Welfare Assurance Number” question.
3. Is proprietary/privileged information included in the application?
This field is required.
Patentable ideas; trade secrets; or privileged, confidential commercial, or financial information
should be included in applications only when such information is necessary to convey an
understanding of the proposed project.
If the application includes such information, check “Yes” and clearly mark each line or paragraph
on the pages containing the proprietary/privileged information with a statement similar to: “The
following contains proprietary/privileged information that (name of applicant) requests not be
released to persons outside the government, except for purposes of review and evaluation.” This
statement can be included at the top of each page as applicable.
If a grant is awarded as a result of or in connection with the submission of this application, the
government shall have the right to use or disclose the information to the extent authorized by law.
Although the grantee institution and the PD/PI will be consulted about any such disclosure, the
NIH and other PHS agencies will make the final determination. Any indication by the applicant that
the application contains proprietary or privileged information does not automatically shield the
information from release in response to a Freedom of Information Act (FOIA) request should the
application result in an award (see 45 CFR 5). Additionally, if an applicant fails to identify
proprietary information at the time of submission as instructed here, a significant substantive
justification will be required to withhold the information if requested under FOIA.
M - 42

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
4. Environmental Questions
Question 4 pertains to the environmental impact of the proposed research.
4.a. Does this Project Have an Actual or Potential Impact - positive or negative - on the envir-
onment?
This field is required.
Indicate whether or not this project has an actual or potential impact on the environment.
Most NIH research grants are not expected to individually or cumulatively have a significant effect
on the environment, and NIH has established several categorical exclusions allowing most
applicants to answer “No” unless a specific FOA indicates that the National Environmental Policy
Act (NEPA) applies. However, if an applicant expects that the proposed project will have an actual
or potential impact on the environment, or if any part of the proposed research and/or project
includes one or more of the following scenarios, check “Yes.”
1. The potential environmental impacts of the proposed research may be of greater scope or
size than other actions included within a category.
2. The proposed research threatens to violate a federal, state, or local law established for the
protection of the environment or for public health and safety.
3. Potential effects of the proposed research are unique or highly uncertain.
4. Use of especially hazardous substances or processes is proposed for which adequate and
accepted controls and safeguards are unknown or not available.
5. The proposed research may overload existing waste treatment plants due to new loads
(volume, chemicals, toxicity, additional hazardous wasted, etc.).
6. The proposed research may have a possible impact on endangered or threatened species.
7. The proposed research may introduce new sources of hazardous/toxic wastes or require
storage of wastes pending new technology for safe disposal.
8. The proposed research may introduce new sources of radiation or radioactive materials.
9. Substantial and reasonable controversy exists about the environmental effects of the
proposed research.
4.b. If yes, please explain:
If you answered “Yes” to Question 4.a., you must provide an explanation here as to the actual or
potential impact of the proposed research on the environment. Your entry is limited to 55
characters.
4.c. If this project has an actual or potential impact on the environment, has an exemption been
authorized or an environmental assessment (EA) or environmental impact statement (EIS) been
performed? Yes/No.
This field is required if you answered “Yes” to Question 4.a. Check “Yes” or “No.”
4.d. If yes, please explain:
Enter additional details about the EA or EIS here. Your entry is limited to 55 characters.
M - 43

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
5. Is the research performance site designated, or eligible to be designated, as a
historic place?
This field is required.
If any research performance site is designated, or eligible to be designated, as a historic place,
check the “Yes” box. Otherwise, check “No.”
5.a. If yes, please explain:
If you checked “Yes” to indicate that any performance site is designated, or eligible to be
designated, as a historic place, provide the explanation here. Your entry is limited to 55 characters.
6. Does this project involve activities outside of the United States or partnerships
with international collaborators?
This field is required.
Indicate whether this project involves activities outside of the United States or partnerships with
international collaborators. Check “Yes” or “No.”
Applicants to NIH and other PHS agencies must check “Yes” if the applicant organization is a
foreign institution or if the project includes a foreign component. See NIH Glossary for a definition
of a foreign component.
If you have checked “Yes” to Question 6, you must include a “Foreign Justification” attachment in
Field 12, Other Attachments. Describe special resources or characteristics of the research project
(e.g., human subjects, animals, disease, equipment, and techniques), including the reasons why the
facilities or other aspects of the proposed project are more appropriate than a domestic setting. In
the body of the text, begin the section with a heading indicating “Foreign Justification” and name
the file “Foreign Justification.”
Additional Instructions for Multi-project:
Overall Component: If the answer to Question 6 is “Yes” for any Other Component,
then you must answer “Yes” for the Overall Component.
6.a. If yes, identify countries:
This field is required if you answered “Yes” to Question 6. Enter the countries with which
international cooperative activities are planned.
You may use abbreviations. Your entry is limited to 55 characters.
6.b. Optional Explanation:
This field is optional. Enter an explanation for involvement with outside entities. Your entry is
limited to 55 characters.
7. Project Summary/Abstract
The "Project Summary/Abstract" attachment is required.
The project summary is a succinct and accurate description of the proposed work and should be
able to stand on its own (separate from the application). This section should be informative to
other persons working in the same or related fields and understandable to a scientifically literate
M - 44
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
reader. Avoid both descriptions of past accomplishments and the use of the first person. Please be
concise.
Format:
This section is limited to 30 lines of text, and must follow the required font and margin
specifications. A summary that exceeds the 30-line limit will be flagged as an error by the Agency
upon submission. Use of hyperlinks and URLs in this section is not allowed unless specified in the
funding opportunity announcement.
Attach this information as a PDF file. See the Format Attachments page.
Content:
State the application's broad, long-term objectives and specific aims, making reference to the
health relatedness of the project (i.e., relevance to the mission of the agency). Describe the
research design and methods for achieving the stated goals. Be sure that the project summary
reflects the key focus of the proposed project so that the application can be appropriately
categorized.
Do not include proprietary, confidential information or trade secrets in the project summary. If the
application is funded, the project summary will be entered into an NIH database and made
available on the NIH Research Portfolio Online Reporting Tool (RePORT) and will become public
information.
Note that the "Project Summary/Abstract" attachment is not same as the "Research Strategy"
attachment.
Additional Instructions for Multi-project:
Overall and Other Components: A project summary is required for both the
Overall Component and all Other Components. Each project summary attachment is
limited to 30 lines of text.
8. Project Narrative
The "Project Narrative" attachment is required.
Content:
Describe the relevance of this research to public health in, at most, three sentences. For example,
NIH applicants can describe how, in the short or long term, the research would contribute to
fundamental knowledge about the nature and behavior of living systems and / or the application
of that knowledge to enhance health, lengthen life, and reduce illness and disability. Use of
hyperlinks and URLs in this section is not allowed unless specified in the funding opportunity
announcement. If the application is funded, this public health relevance statement will be
combined with the project summary (above) and will become public information.
Additional Instructions for Multi-project:
Overall Component: The "Project Narrative" attachment is required.
Other Components: Refer to the specific FOA to determine whether the "Project
Narrative" attachment is required for any Other Components. Note: The form may
M - 45
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
show ‘*’ indicating it is a required field, but it is only required for the Overall
Component and the ‘*’ can be ignored for Other Components.
9. Bibliography & References Cited
Who must complete the “Bibliography & References Cited” attachment:
The “Bibliography & References Cited” attachment is required unless otherwise noted in the FOA.
Format:
Attach this information as a PDF file. See the Format Attachments page. Use of hyperlinks and
URLs in this section is not allowed unless specified in the funding opportunity announcement.
Content:
See the following instructions for which references to include in the “Bibliography and References
Cited” attachment.
Additional Instructions for Multi-project:
Overall and Other Components: The “Bibliography & References Cited”
attachment should include any references cited in M.400 - PHS 398 Research Plan
Form and in the M.500 - PHS Human Subjects and Clinical Trials Information form.
When citing articles that fall under the Public Access Policy, were authored or co-authored by the
applicant, and arose from NIH support, provide the NIH Manuscript Submission reference number
(e.g., NIHMS97531) or the PubMed Central (PMC) reference number (e.g., PMCID234567) for each
article. If the PMCID is not yet available because the Journal submits articles directly to PMC on
behalf of their authors, indicate “PMC Journal – In Process.” NIHmaintains a list of such journals.
Citations that are not covered by the Public Access Policy, but are publicly available in a free,
online format may include URLs or PubMed ID (PMID) numbers along with the full reference.
Active hyperlinks in this section are not allowed. The references should be limited to relevant and
current literature. While there is not a page limitation, it is important to be concise and to select
only those literature references pertinent to the proposed research.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions for more information.
Additional Instructions for Multi-project:
Overall and Other Components:Unless specific instructions are provided in the
FOA, applicants have the option of including the "Bibliography & References Cited"
attachment in the Overall Component, Other Components, or both. User-defined
bookmarks provided in the Bibliography & References Cited attachment will be
included with the bookmarks of the assembled application image in eRA Commons.
If you include the “Bibliography & References Cited” attachment only in the Overall
Component, you may want to use bookmarks to organize references by component.
M - 46

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
10. Facilities & Other Resources
Format:
The “Facilities & Other Resources” attachment is required unless otherwise specified in the FOA.
Use of URLs and hyperlinks in this section is not allowed unless specified in the funding
opportunity announcement.
Content:
Describe how the scientific environment in which the research will be done contributes to the
probability of success (e.g., institutional support, physical resources, and intellectual rapport). In
describing the scientific environment in which the work will be done, discuss ways in which the
proposed studies will benefit from unique features of the scientific environment or from unique
subject populations or how studies will employ useful collaborative arrangements.
If there are multiple performance sites, describe the resources available at each site.
Describe any special facilities used for working with biohazards and any other potentially
dangerous substances. Note: Information about select agents must be described in the
Research Plan, Select Agent Research.
For early stage investigators (ESIs), describe institutional investment in the success of the
investigator. See NIH's Early Stage Investigator (ESI) Policies. Your description may include the
following elements:
l
resources for classes, travel, or training;
l
collegial support, such as career enrichment programs, assistance and guidance in the
supervision of trainees involved with the ESI’s project, and availability of organized peer
groups;
l
logistical support, such as administrative management and oversight and best practices
training;
l
financial support, such as protected time for research with salary support.
Additional Instructions for Multi-project:
Unless specific instructions are provided in the FOA, applicants have the option of
including the "Facilities & Other Resources" attachment in the Overall Component,
Other Components, or both.
11. Equipment
The “Equipment” attachment is required.
Format:
Attach this information as a PDF file. Use of URLs and hyperlinks in this section is not allowed
unless specified by the funding opportunity announcement.
Content:
List major items of equipment already available for this project and, if appropriate, identify the
equipment's location and pertinent capabilities.
M - 47

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.220 - R&R Other Project Information Form
Additional Instructions for Multi-project:
Unless specific instructions are provided in the FOA, applicants have the option of
including the "Equipment" attachment in the Overall Component, Other
Components, or both (whichever is most appropriate for your application). User-
defined bookmarks provided in the Equipment attachment will be included with the
bookmarks of the assembled application image in eRA Commons. If you include the
“Equipment” attachment only in the Overall Component, you may want to use
bookmarks to organize equipment by component.
12. Other Attachments
Attach a file to provide additional information only in accordance with the FOA and/or agency-
specific instructions.
If applicable, attach a “Foreign Justification” here. (See Question 6 above).
M - 48

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.230 - Project/Performance Site Location(s) Form
M.230 - Project/Performance Site
Location(s) Form
The Project/Performance Site Location(s) Form is used
for all grant applications. It is used to report the primary
location and any other locations at which the project will
be performed.
View larger image
Quick Links
Project/Performance Site Primary Location
Project/Performance Site Location 1
Additional Location(s)
Using the Project/Performance Site Location(s) Form:
This form allows for the collection of multiple performance sites. If you need to add more
project/performance site locations than the form allows, enter the information in a separate file and add
it to the “Additional Locations” section.
Project/Performance Site Primary Location
Generally, the primary location should be that of the applicant organization or identified as off-site
in accordance with the conditions of the applicant organization’s negotiated Facilities and
Administrative (F&A) agreement. This information must agree with the F&A information on the
budget form of the application.
Provide an explanation of resources available from each project/performance site on the "Facilities
and Resources" attachment of the M.220 - R&R Other Project Information Form.
If the proposed project involves human subjects or live vertebrate animals, it is up to the applicant
organization to ensure that all sites meet certain criteria:
Human Subjects: If a project/performance site is engaged in research involving human subjects,
the applicant organization is responsible for ensuring that the project/performance site operates
under an appropriate Federal Wide Assurance for the protection of human subjects and complies
with 45 CFR 46 and other NIH human subject related policies described in the NIH Grants Policy
Statement, Section 4.1.15: Human Subjects Protections.
Vertebrate Animals: For research involving live vertebrate animals, the applicant organization
must ensure that all project/performance sites hold an Office of Laboratory Animal Welfare
(OLAW)-approved Animal Welfare Assurance. If the animal work will be conducted at an institution
with an Animal Welfare Assurance and the applicant organization does not have the following:
l
an animal care and use program;
l
facilities to house animals and conduct research on site; and
M - 49

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.230 - Project/Performance Site Location(s) Form
l
an IACUC;
then applicant must obtain an Inter-institutional Assurance from OLAW prior to an award.
Additional Instructions for Multi-project:
Overall Component: Include only the primary site for the entire application, which
is typically the applicant organization.
Other Components: List the primary site for the component, which is typically the
lead organization of the component. Describe any consortium/contractual
arrangements in the "Consortium/Contractual Arrangements" attachment in M.400
– PHS 398 Research Plan Form.
“I am submitting an application as an individual, and not on behalf of a company, state, local or
tribal government, academia, or other type of organization”:
Do not check the box for “I am submitting an application as an individual, and not on behalf of a
company, state, local or tribal government, academia, or other type of organization” unless
otherwise specified by the FOA.
Organization Name:
This field is required. Enter the organization name of the primary site where the work will be
performed.
Unique Entity Identifier (UEI):
This field is required for the primary performance site.
Enter the UEI associated with the organization where the project will be performed.
Street1:
This field is required. Enter the first line of the street address of the primary performance site
location.
Street2:
Enter the second line of the street address of the primary performance site location.
City:
This field is required. Enter the city for the address of the primary performance site location.
County:
Enter the county of the primary performance site location.
State:
This field is required if the site is located in the United States or its Territories. Enter the state or
territory where the primary performance site is located.
Province:
If “Country" is Canada, enter the province for the primary performance site; otherwise, skip the
“Province” field.
Country:
This field is required. Select the country of the address for the primary performance site location.
M - 50

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.230 - Project/Performance Site Location(s) Form
ZIP/Postal Code:
The ZIP+4 is required if the primary performance site location is in the United States. Otherwise,
the postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the primary
performance site.
Project/Performance Site Congressional District:
Enter the Congressional District as follows: a 2-character state abbreviation, a hyphen, and a 3-
character district number. Examples: CA-005 for California’s 5th district, VA-008 for Virginia’s 8th
district.
It is likely this field will be identical to the “Congressional District of Applicant” field provided
elsewhere in the application.
If the program/project is outside the United States, enter 00-000.
For States and U.S. territories with only a single congressional district, enter “001” for the district
number.
For jurisdictions with no representative, enter “099.”
For jurisdictions with a nonvoting delegate, enter “098” for the district number. Example: DC-098
or PR-098.
If all districts in a state are affected, enter “all” for the district number. Example: "MD-all" for all
congressional districts in Maryland.
If nationwide (all districts in all states), enter "US-all."
If you do not know the Congressional District: Go to the United States House of
Representatives website and search for the Congressional District by entering the ZIP+4. If you do
not know the ZIP+4, look it up on the USPS Look Up Zip Code website.
Project/Performance Site Location 1
Use this “Project/Performance Site Location 1” block to provide information on performance sites
in addition to the Primary Performance Site listed above, if applicable. Include any VA facilities and
foreign sites.
Additional Instructions for Multi-project:
Other Components: List all performance sites that apply to the specific component.
Organization Name:
Enter the organization name of the performance site location.
Unique Entity Identifier (UEI):
Enter the UEI associated with the performance site.
Street1:
This field is required. Enter first line of the street address of the performance site location.
Street2:
Enter the second line of the street address of the performance site location.
M - 51

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.230 - Project/Performance Site Location(s) Form
City:
This field is required. Enter the city for the address of the performance site location.
County:
Enter the county of the performance site location.
State:
This field is required if the project performance site is located in the United States or its Territories.
Enter the state or territory where the performance site is located.
Province:
If “Country” is Canada, enter the province for the performance site; otherwise, skip the “Province”
field.
Country:
This field is required. Select the country of the performance site location.
ZIP/Postal Code:
The ZIP+4 is required if the performance site location is in the United States. Otherwise, the postal
code is optional. Enter the ZIP+4 (nine-digit postal code) of the performance site location.
Project/Performance Site Congressional District:
Enter the Congressional District as follows: a 2-character state abbreviation, a hyphen, and a 3-
character district number. Examples: CA-005 for California’s 5th district, VA-008 for Virginia’s 8th
district.
If the program/project is outside the United States, enter 00-000.
For States and U.S. territories with only a single congressional district enter “001” for the district
number.
For jurisdictions with no representative, enter “099.”
For jurisdictions with a nonvoting delegate, enter “098” for the district number. Example: DC-098
or PR-098.
If all districts in a state are affected, enter “all” for the district number. Example: "MD-all" (for all
congressional districts in Maryland).
If nationwide (all districts in all states), enter "US-all."
If you do not know the Congressional District: Go to the United States House of
Representatives website and search for your Congressional District by entering your ZIP+4. If you
do not know the ZIP+4 look it up on the USPS Look Up Zip Code website.
Additional Location(s)
If you need to add more project/performance site locations than the form allows, enter the
information in a separate file and add it to the “Additional Locations” section.
A format page for Additional Performance Sites can be found on NIH's Additional Performance
Site Format Page.
M - 52

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
M.240 - R&R Senior/Key Person Profile
(Expanded) Form
The R&R Senior/Key Person Profile (Expanded) Form is
used for all grant applications, and allows the collection of
data for all senior/key persons associated with the project.
Some information for the PD/PI may be pre-populated
from the SF424 (R&R) form. See instructions in M.200 - SF
424 (R&R) Form if these fields are empty.
View larger image
Quick Links
Profile - Project Director/Principal Investigator
Instructions for a Biographical Sketch
Profile - Senior/Key Person
Additional Senior/Key Person Profile(s)
Using the R&R Senior/Key Person Profile (Expanded) Form
This form allows for the data collection for a PD/PI and up to 99 other senior/key individuals (including
any multi-PD/PIs). After the first 100 individuals have been entered, use the “Additional Senior/Key
Person Profiles Format Page” to attach any remaining data.
To ensure proper performance of this form, save your work frequently.
Who qualifies as a Senior/Key Person?
Unless otherwise specified in a FOA, senior/key personnel are defined as all individuals who contribute
in a substantive, meaningful way to the scientific development or execution of the project, whether or
not salaries are requested. Consultants should be included in this “Senior/Key Person Profile
(Expanded)” Form if they meet this definition.
List individuals that meet the definition of senior/key regardless of what organization they work for.
Profile - Project Director/Principal Investigator
Enter data in this “Profile – Project Director/Principal Investigator” section for the Project
Director/Principal Investigator (PD/PI).
The PD/PI must have an eRA Commons account with the PI role, and the account must be affiliated
with the applicant organization. If you are proposing research at an institute other than the one
you are currently at, do not create a separate Commons account with the proposed applicant
organization. For information on eRA Commons account administration, see the eRA Account
Management System’s Online Help.
M - 53

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
Special Instructions for Multiple PD/PIs: When submitting an application involving multiple
PD/PIs, list the “Contact” PD/PI in this field. List all additional PD/PIs in the Senior/Key Person
section(s) below.
Additional Instructions for Multi-project:
Overall Component: List the PD/PI (or Contact PD/PI if submitting a multi-PD/PI
application) for the entire application.
Other Components: List the component lead.
Prefix:
This field may be pre-populated from the SF 424 (R&R) and reflects the prefix, if applicable, for the
name of the PD/PI.
First Name:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the first
(given) name of the PD/PI.
Middle Name:
This field may be pre-populated from the SF 424 (R&R) and reflects the middle name of the PD/PI.
Last Name:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the last
(family) name of the PD/PI.
Suffix:
This field may be pre-populated from the SF 424 (R&R) and reflects the suffix for the name of the
PD/PI.
Position/Title:
This field may be pre-populated from the SF 424 (R&R) and reflects the position/title of the PD/PI.
Department:
This field may be pre-populated from the SF 424 (R&R) and reflects the name of the primary
organizational department, service, laboratory, or equivalent level within the organization of the
PD/PI.
Organization Name:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the name
of the organization of the PD/PI.
Division:
This field may be pre-populated from the SF 424 (R&R) and reflects the name of the primary
organizational division, office, major subdivision, or equivalent level within the organization of the
PD/PI.
Street1:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the first
line of the street address for the PD/PI.
M - 54

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
Street2:
This field may be pre-populated from the SF 424 (R&R) and reflects the second line of the street
address for the PD/PI.
City:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the city
for the address of the PD/PI.
County/Parish:
This field may be pre-populated from the SF 424 (R&R) and reflects the county/parish for the
address of the PD/PI.
State:
This field is required if the PD/PI is located in the United States or its Territories. This field may be
pre-populated from the SF 424 (R&R) and reflects the state or territory in which the PD/PI is
located.
Province:
If “Country” is Canada, enter the province for the PD/PI; otherwise, skip the “Province” field. This
field may be pre-populated from the SF 424 (R&R) and reflects the province in which the PD/PI is
located.
Country:
This field may be pre-populated from the SF 424 (R&R) and reflects the country for the address of
the PD/PI.
ZIP/Postal Code:
The ZIP+4 is required if the PD/PI address is in the United States. Otherwise, the postal code is
optional. This field may be pre-populated from the SF 424 (R&R) and reflects the postal code of
the address of the PD/PI.
Phone Number:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the
daytime phone number for the PD/PI.
Fax Number:
This field may be pre-populated from the SF 424 (R&R) and reflects the fax number for the PD/PI.
E-mail:
This field is required. This field may be pre-populated from the SF 424 (R&R) and reflects the e-
mail address for the PD/PI.
Credential, e.g., agency login:
This field is required. Enter the assigned eRA Commons username for the project’s PD/PI. The eRA
Commons username must hold the PI role and be affiliated with the applicant organization.
Applications will not pass agency validation requirements without a valid eRA Commons
username.
Special Instructions for Multiple PD/PI: The Commons username must be provided for
all individuals assigned the Project Role of PD/PI on the application.
M - 55

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
Project Role:
Enter "PD/PI" for the Project Role for the PD/PI.
Additional Instructions for Multi-project:
Other Components: For the “Profile – Project Director/Principal Investigator”
section, enter “Other (Specify)” and enter “Project Lead” for the “Other Project Role
Category” field, unless otherwise specified in the FOA. The PD/PI role is used only in
the Overall Component.
Other Project Role Category:
Skip the “Other Project Role Category” field, as no other role can be added to the PD/PI role.
Degree Type:
Enter the highest academic or professional degree or other credentials (e.g., R.N.).
Degree Year:
Enter the year the highest degree or other credential was obtained.
Attach Biographical Sketch
Provide a biographical sketch for each PD/PI. See instructions below on how to complete a
biographical sketch.
Attach Current & Pending Support:
Do not use this attachment upload for NIH and other PHS agency submissions unless otherwise
specified in the FOA.
While this information is not required at the time of application submission, it may be requested
later in the pre-award cycle. If and when this occurs, refer to the NIH Grants Policy Statement,
Section 2.5.1: Just-in-Time Procedures.
Instructions for a Biographical Sketch
These instructions apply to Research (R), Career Development (K), Training (T), Fellowship (F),
Multi-project (M), and SBIR/STTR (B). Hyperlinks and URLs are only allowed when specifically noted
in funding opportunity announcement (FOA) and form field instructions.
Who must complete the “Biographical Sketch” section:
All senior/key personnel and other significant contributors (OSCs) must include biographical
sketches (biosketches).
Format:
Use the sample format on the Biographical Sketch Format Page to prepare this section for all grant
applications.
Figures, tables (other than those included in the provided format pages), or graphics are not
allowed in the biosketch. Do not embed or attach files (e.g. video, graphics, sound, data).
The biosketch may not exceed five pages per person. This five-page limit includes the table at the
top of the first page.
Attach this information as a PDFfile. See the Format Attachments page.
M - 56

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
Content:
Note that the instructions here follow the format of Biographical Sketch Format Page.
Name:
Fill in the name of the senior/key person or other significant contributor in the “Name” field of the
Biosketch Format Page.
eRA Commons User Name:
If the individual is registered in the eRA Commons, fill in the eRA Commons User Name in the “eRA
Commons User Name” field of the Biosketch Format Page.
The “eRA Commons User Name” field is required for the PD/PI (including career development and
fellowship applicants), primary sponsors of fellowship applicants, all mentors of candidates for
mentored career development awards, and candidates for diversity and reentry research
supplements.
The “eRA Commons User Name” field is optional for other project personnel.
The eRA Commons User Name should match the information provided in the Credential
field of the R&R Senior/Key Person Profile (Expanded) Form in your grant application.
Position Title:
Fill in the position title of the senior/key person or other significant contributor in the “Position
Title” field of the Biosketch Format Page.
Education/Training
Complete the education block. Begin with the baccalaureate or other initial professional
education, such as nursing. Include postdoctoral, residency, and clinical fellowship training, as
applicable, listing each separately.
For each entry provide:
l
the name and location of the institution
l
the degree received (if applicable)
l
the month and year of end date (or expected end date). For fellowship applicants only,
also include the month and year of start date.
l
the field of study (for residency entries, the field of study should reflect the area of
residency training)
Following the education block, complete Sections A-D of the biographical sketch.
A. Personal Statement
Briefly describe why you are well-suited for your role(s) in this project. Relevant factors may
include: aspects of your training; your previous experimental work on this specific topic or related
topics; your technical expertise; your collaborators or scientific environment; and/or your past
performance in this or related fields, including ongoing and completed research projects from the
past three years that you want to draw attention to (previously captured under Section D. Research
Support).
You may cite up to four publications or research products that highlight your experience and
qualifications for this project. Research products can include, but are not limited to, audio or video
products; conference proceedings such as meeting abstracts, posters, or other presentations;
patents; data and research materials; databases; educational aids or curricula; instruments or
M - 57

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
equipment; models; protocols; and software or netware. Use of hyperlinks and URLs to cite these
items is not allowed.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions for more information.
Note the following additional instructions for ALL applicants/candidates:
l
If you wish to explain factors that affected your past productivity, such as family care
responsibilities, illness, disability, or military service, you may address them in this “A.
Personal Statement” section.
l
Indicate whether you have published or created research products under another name.
l
You may mention specific contributions to science that are not included in Section C. Do
not present or expand on materials that should be described in other sections of this
Biosketch or application.
l
Figures, tables, or graphics are not allowed.
Note the following instructions for specific subsets of applicants/candidates:
l
For institutional research training, institutional career development, or research education
grant applications, faculty who are not senior/key persons are encouraged, but not
required, to complete the "A. Personal Statement" section.
l
Applicants for dissertation research awards (e.g., R36) should, in addition to addressing
the points noted above, also include a description of their career goals, their intended
career trajectory, and their interest in the specific areas of research designated in the FOA.
l
Candidates for research supplements to promote diversity in health-related research
should, in addition to addressing the points noted above, also include a description of
their general scientific achievements and/or interests, specific research objectives, and
career goals. Indicate any current source(s) of educational funding.
B. Positions, Scientific Appointments and Honors
List in reverse chronological order all current positions and scientific appointments both domestic
and foreign, including affiliations with foreign entities or governments. This includes titled
academic, professional, or institutional appointments whether or not remuneration is received,
and whether full-time, part-time, or voluntary (including adjunct, visiting, or honorary). High
school students and undergraduates may include any previous positions. For individuals who are
not currently located at the applicant organization, include the expected position at the applicant
organization and the expected start date.
List any relevant academic and professional achievements and honors. In particular:
l
Students, postdoctorates, and junior faculty should include scholarships, traineeships,
fellowships, and development awards, as applicable.
l
Clinicians should include information on any clinical licensures and specialty board
certifications that they have achieved.
M - 58

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
C. Contributions to Science
Who should complete the “Contributions to Science” section:
All senior/key persons should complete the “Contributions to Science” section except candidates
for research supplements to promote diversity in health-related research who are high school
students, undergraduates, and post-baccalaureates.
Format:
Briefly describe up to five of your most significant contributions to science. The description of each
contribution should be no longer than one half page, including citations.
While all applicants may describe up to five contributions, graduate students and postdoctorates
may wish to consider highlighting two or three they consider most significant.
Content:
For each contribution, indicate the following:
l
the historical background that frames the scientific problem;
l
the central finding(s);
l
the influence of the finding(s) on the progress of science or the application of those
finding(s) to health or technology; and
l
your specific role in the described work.
l
Figures, tables, or graphics are not allowed.
For each contribution, you may cite up to four publications or research products that are relevant
to the contribution. If you are not the author of the product, indicate what your role or
contribution was. Note that while you may mention manuscripts that have not yet been accepted
for publication as part of your contribution, you may cite only published papers to support each
contribution. Research products can include audio or video products (see the NIH Grants Policy
Statement, Section 2.3.7.7: Post-Submission Grant Application Materials); conference proceedings
such as meeting abstracts, posters, or other presentations; patents; data and research materials;
databases; educational aids or curricula; instruments or equipment; models; protocols; and
software or netware. Use of hyperlinks and URLs to cite these items is not allowed.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions for more information.
You may provide a hyperlinked URL to a full list of your published work. This hyperlinked URL must
be to a Federal Government website (a .gov suffix). NIH recommends using My Bibliography.
Providing a URL to a list of published work is not required.
Descriptions of contributions may include a mention of research products under development,
such as manuscripts that have not yet been accepted for publication. These contributions do not
have to be related to the project proposed in this application.
D. Scholastic Performance
* Note that only the following types of applicants must complete this section:
o
applicants for predoctoral and postdoctoral fellowships
o
applicants to dissertation research grants (e.g., R36)
M - 59

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
o
candidates for research supplements to promote diversity in health-related research
from the undergraduate through postdoctoral levels
Scholastic Performance
Predoctoral applicants/candidates (including undergraduates and post-baccalaureates): List
by institution and year all undergraduate and graduate courses, with grades. In addition, explain
any grading system used if it differs from a 1-100 scale; an A, B, C, D, F system; or a 0-4.0 scale.
Also indicate the levels required for a passing grade.
Postdoctoral applicants: List by institution and year all graduate scientific and/or professional
courses with grades. In addition, explain any grading system used if it differs from a 1-100 scale; an
A, B, C, D, F system; or a 0-4.0 scale. Also indicate the levels required for a passing grade.
Additional Instructions for Multi-project:
Each Senior/Key Person, including the PD/PI, is allowed one biosketch for the entire
application. If an individual will participate on multiple components, attach the
biosketch to any single component.
Profile – Senior/Key Person 1
Enter data in this “Profile – Senior/Key Person 1” section to provide information on a senior/key
person (other than the PD/PI listed above), if applicable.
Format:
List all senior/key person profiles, followed by other significant contributors (OSC) profiles.
Content – Who to include in the “Profile – Senior/Key Person” section:
Senior/Key Persons: Fill in a separate “Profile – Senior/Key Person” block for each senior/key
personnel. Those with a postdoctoral role should be included if they meet the NIH Glossary
definition of senior/key personnel. A biosketch is required for all senior/key persons.
Other Significant Contributors: Also use the “Profile – Senior/Key Person” section to list any
other significant contributors (OSCs). Consultants should be included if they meet the NIH
Glossary definition of OSC. OSCs should be listed after all other senior/key persons.
A biosketch is required for all OSCs. The biosketch should highlight the OSC’s accomplishments as
a scientist. Reviewers assess these pages during peer review. For more information on review
criteria, see the Review Criteria at a Glance document. Although Other Support information is
required as a just-in-time submission, Other Support information will NOT be required or accepted
for OSCs since considerations of overlap do not apply to these individuals.
Should the level of involvement increase for an individual listed as an OSC, thus requiring
measurable effort on the award, the individual must be redesignated as “senior/key personnel.”
This change must be made before any compensation is charged to the project.
For more information:
For more information, refer to these NIHSenior/Key Personnel Frequently Asked Questions.
Prefix:
Enter or select the prefix, if applicable, for the name of the senior/key person.
M - 60

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
First Name:
This field is required. Enter the first (given) name of the senior/key person.
Middle Name:
Enter the middle name of the senior/key person.
Last Name:
This field is required. Enter the last (family) name of the senior/key person.
Suffix:
Enter or select the suffix, if applicable, for the senior/key person.
Position/Title:
Enter the position/title of the senior/key person.
Department:
Enter the name of the primary organizational department, service, laboratory, or equivalent level
within the organization of the senior/key person.
Organization Name:
This field is required. Enter the name of the organization of the senior/key person.
Division:
Enter the name of the primary organizational division, office, major subdivision, or equivalent level
within the organization of the senior/key person.
Street1:
This field is required. Enter the first line of the street address for the senior/key person.
Street2:
Enter the second line of the street address for the senior/key person.
City:
This field is required. Enter the city for the address of the senior/key person.
County/Parish:
Enter the county/parish for the address of the senior/key person.
State:
This field is required if the Senior/Key person is located in the United States or its Territories. Enter
the state or territory where the senior/key person is located.
Province:
If “Country” is Canada, enter the province for the senior/key person; otherwise, skip the “Province”
field.
Country:
This field is required. Select the country for the address of the senior/key Person.
ZIP/Postal Code:
The ZIP+4 is required if the Senior/Key Person is in the United States. Otherwise, the postal code is
optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the senior/key person.
M - 61
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
Phone Number:
This field is required. Enter the daytime phone number for the senior/key person.
Fax Number:
Enter the fax number for the senior/key person.
E-mail:
This field is required. Enter the e-mail address for the senior/key person.
Credential, e.g., agency login:
This field is required. Applies to Senior/Key Personnel as defined in the NIH Grants Policy
Statement (NIH GPS 1.2) as well as Other Significant Contributors (OSCs). Enter the assigned eRA
Commons username for the senior / key Person.
Project Role:
Select a project role. Use "Other (Specify)" if the desired category is not available.
Special Instructions for Multiple PD/PIs:All PD/PIs must be assigned the “PD/PI” role,
even those at organizations other than the applicant organization. The role of “Co-PD/PI”
is not currently used by NIH or other PHS agencies to designate a multiple PD/PI
application. In order to avoid confusion, do not use the role of “Co-PD/PI.”
Note on OSCs: For OSCs, enter “Other (Specify)” for the “Project Role” field, and enter “Other
Significant Contributor” in the “Other Project Role Category” field.
Other Project Role Category:
Complete this field (e.g., Engineer, Chemist, Sponsor, Mentor) if you selected “Other Professional”
or “Other (Specify)” in the “Project Role” field.
Degree Type:
Enter the highest academic or professional degree or other credentials (e.g., R.N.).
Degree Year:
Enter the year the highest degree or other credential was obtained.
Attach Biographical Sketch:
Provide a biographical sketch for each senior/key person and each OSC. See instructions above on
how to complete a biographical sketch.
Attach Current & Pending Support:
Note: The terms “current and pending support,” “other support,” and “active and pending
support” are used interchangeably.
Do not use the "Current &Pending Support" attachment upload for NIH or other PHS agency
submissions unless otherwise specified in the FOA.
While this information is not required at the time of application submission, it may be requested
later in the pre-award cycle. If and when this occurs, refer to the NIH Grants Policy Statement,
Section 2.5.1: Just-in-Time Procedures for instructions and use the Current and Pending Support
Format Page.
M - 62

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.240 - R&RSenior/Key Person Profile (Expanded) Form
Additional Senior / Key Person Profile(s)
If you need to add more Senior/Key Person Profiles than the form allows, enter the information in
a separate file and attach it as a PDF.
A format page for Additional Senior/Key Person Profiles can be found at NIH's Additional
Senior/Key Person Form page.
M - 63
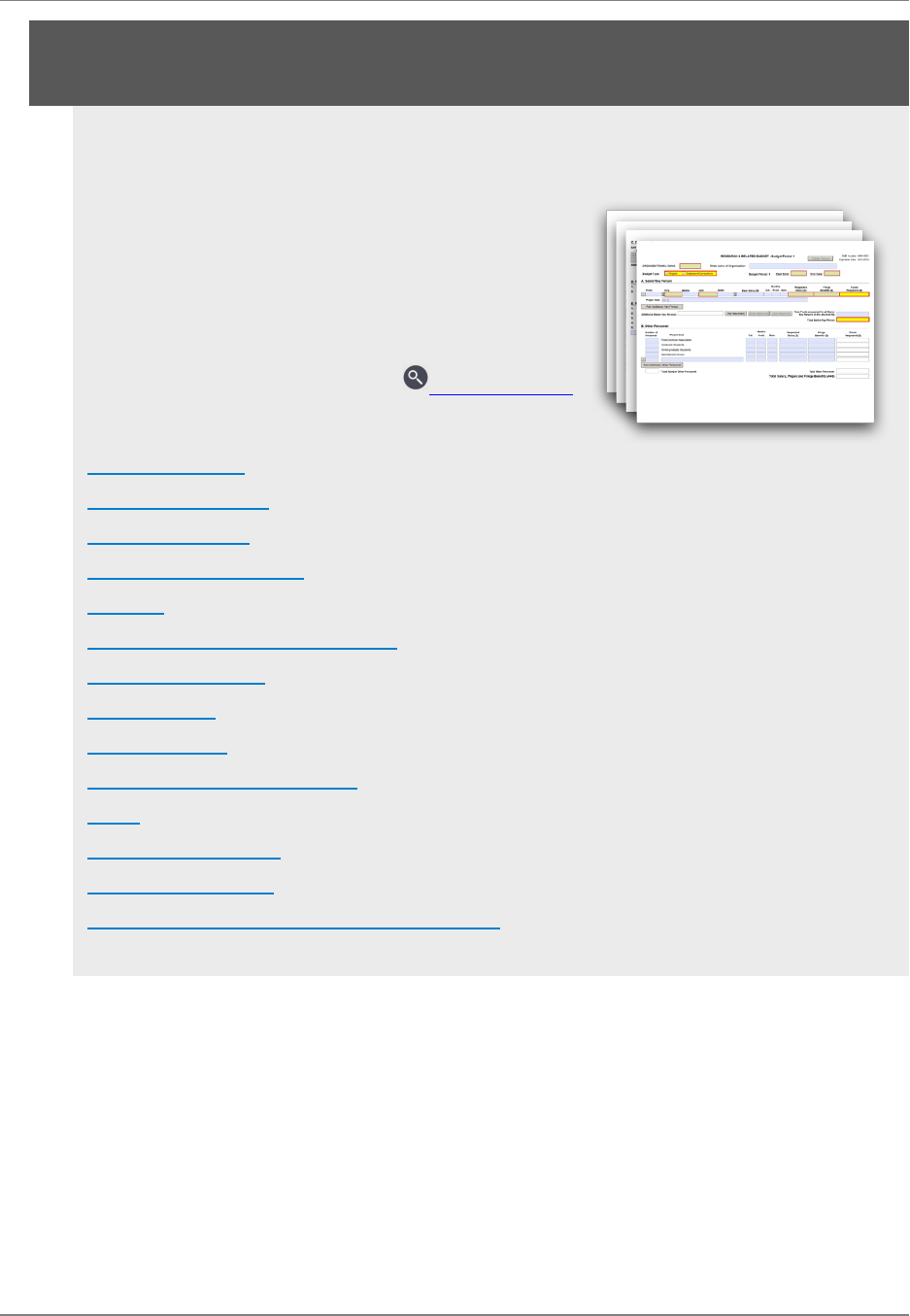
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
M.300 - R&R Budget Form
The R&R Budget Form is used in the majority of
applications; however, it is important to refer to your
specific FOA for guidance on which budget form(s) are
allowed for your application.
Some application forms packages include two optional
budget forms — (1) the R&R Budget Form and, (2) PHS
398 Modular Budget Form. Include only one of these
forms, but not both, in your application.
View larger image
Quick Links
Introductory Fields
A. Senior/Key Person
B. Other Personnel
C. Equipment Description
D. Travel
E. Participant/Trainee Support Costs
F. Other Direct Costs
G. Direct Costs
H. Indirect Costs
I. Total Direct and Indirect Costs
J. Fee
K. Total Costs and Fee
L. Budget Justification
Research & Related Budget - Cumulative Budget
Who should use the R&R Budget Form?
There are two primary types of Budget Forms: detailed R&R and PHS 398 modular. Generally, you must
use the R&R Budget Form if you are applying for more than $250,000 per budget period in direct costs,
and you must use the Modular Budget Form if you are applying for less than $250,000. However, some
grant mechanisms or programs (e.g., training grants) may require other budget forms to be used. Refer
to your FOA and to the following instructions for guidance on which Budget Form to use.
Note: The terms “detailed budget” and “R&R Budget” are used interchangeably.
If you are requesting a budget with $500,000 or more in direct costs for any budget period, contact the
awarding component to determine whether you must obtain prior approval before submitting the
M - 64

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
application. For more information on applications that request $500,000 or more in direct costs, see the
NIH Grants Policy Statement, Section 2.3.7.2: Acceptance for Review of Unsolicited Applications
Requesting $500,000 or More in Direct Costs.
Special Instructions for Foreign Organizations (Non-domestic [non-U.S.] Entities): All
competing (new, renewal, resubmission, and revision) grant applications from foreign (non-U.S.)
institutions must use the R&R Budget Form. Do not use the PHS 398 Modular Budget Form. For
additional information, see NIH Guide Notice on the Requirement for Detailed Budget Submissions
from Foreign Institutions and the NIH Grants Policy Statement, Section 13.3.1: Budget. Applications
from foreign organizations must request budgets in U.S. dollars.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) is included in the proposed application, you must use the R&R Budget Form and cannot
use the PHS Modular Budget Form, regardless of the activity code. Whether or not you incur costs to
obtain HFT, you will need to include a “Human Fetal Tissues Costs” line item (F.8-17) and a Budget
Justification attachment (L).
Note on Subawards/Consortiums: If you have a subaward/consortium, you must use the R&R
Subaward Budget Attachment(s) Form in conjunction with the R&R Budget Form. The prime must
extract the R&R Subaward Budget Attachment(s) from the R&R Subaward Budget Attachment(s) Form
and send the extracted file to the subaward/consortium. The consortium should complete the R&R
Subaward Budget Attachment, following the instructions here and in M.310 – R&R Subaward Budget
Attachment(s) Form.
For more information:
For more information on how to prepare your budget, see NIH's Develop Your Budget page.
Additional Instructions for Multi-project:
Developing a Multi-project Budget: The structure of a Multi-project application
reflects where the work will be done and not necessarily the flow of funds. If most of
the work for a particular component is done by a collaborating organization, then
that organization can be set up as the lead organization for that component.
The main budget form for the component must reflect the Unique Entity Identifier
(UEI) for the lead organization and Project for the Budget Type. If the applicant
organization is responsible for a portion of the work for that component, then their
costs would be reflected on a Subaward Budget Form with the applicant
organization UEI and Subaward / Consortium for the Budget Type. Subaward Budget
Forms simply record budget data. They do not indicate that funds must flow
through the lead organization for the component.
The UEI on each budget form is used to identify the budget data associated with
each organization. When the UEI on the budget form is the same as the UEI on the
Overall Component’s SF424 R&R form, the budget data is associated with the
applicant organization. When the UEI is different, it is seen as belonging to a
subaward.
For more information, refer to NIH's Frequently Asked Questions on Applying
Electronically.
M - 65
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
Overall Component: Most budget data is collected within the Other Components.
Complete only the M.200 - SF 424 (R&R) Form, Estimated Project Funding section
and the M.350 - PHS Additional Indirect Costs Form (if applicable). The PHS
Additional Indirect Costs Form is used to gather any additional information
allowable under the grantee's negotiated F&A rate agreement needed to calculate
the F&A rate for the Overall Component's first $25,000 on each subaward that leads
an entire component. The PHS Additional Indirect Costs Form should not be used
when all components are led by the applicant organization.
System-generated budget summaries (including a Composite Application Budget
Summary) based on budget data collected within the Other Components are
included in the summaries section of the assembled application image.
Budget summaries will:
l
appear in the Overall section of the assembled application image in eRA
Commons;
l
will be compiled from R&R budget data collected in the Other Components;
and
l
will be generated upon submission.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue:
l
If the use of human fetal tissue obtained from elective abortions (HFT) (as defined
in the NIH Grants Policy Statement) is included in the proposed application, you
must provide HFT budget information in the component(s) where the research
involving HFT is conducted.
Special Instructions for Applications Submitted with a Data Management
and Sharing Plan:
Overall Component: The "Data Management and Sharing Plan" attachment must be
provided within the PHS 398 Research Plan Form in the Overall Component. Do not
include budget information for Data Management and Sharing Costs in the Overall
Component.
Other Components: Include budget information for Data Management and Sharing
Costs within the applicable component(s). Do not include a “Data Management and
Sharing Plan” attachment within the components. Any component-specific
information should be described within the overall “Data Management and Sharing
Plan” attachment provided within the PHS 398 Research Plan Form in the Overall
Component.
Using the R&R Budget Form:
The location of the R&R Budget Form may vary with the type of submission (e.g., under an “Optional
Forms” tab).
You must complete a separate detailed budget for each budget period requested. The form will
generate a cumulative budget for the total project period. If no funds are requested for a required field,
enter “0.”
You must round to the nearest whole dollar amount in all dollar fields.
Competing Revision Applications: For a supplemental/revision application, complete fields for which
additional funds are requested in addition to all required fields. If the initial budget period of the
M - 66

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
supplemental/revision application is less than 12 months, prorate the personnel costs and other
appropriate items of the detailed budget.
Introductory Fields
Unique Entity Identifier (UEI):
This field is required. This field may be pre-populated and should reflect the UEI of the applicant
organization (or of the lead organization for the component of a multi-project application).
Enter name of Organization:
This field may be pre-populated. Enter the name of the organization.
Budget Type:
This field is required. Check the appropriate box for your budget type, following these guidelines:
l
Project: The budget being requested is for the primary applicant organization.
l
Subaward/Consortium: The budget being requested is for subaward/consortium
organization(s). Note, separate budgets are required only for subaward/consortium
organizations that perform a substantive portion of the project. For
subawards/consortiums that do not perform a substantive portion of the project, then
you must include their costs in Field F5. Subawards/Consortium/Contractual Costs and in
the prime’s Section L. Budget Justification.
If you are preparing an application that includes a subaward/consortium that performs a
substantive portion of the project, in addition to completing this form, see also the instructions for
M.310 - R&R Subaward Budget Attachment(s) Form.
Additional Instructions for Multi-project:
Project: The budget being requested is for the organization leading the component.
Subaward/Consortium: The budget being requested is for other organizations
performing work for the component. When the applicant organization is
participating on a component, but not leading that component, their costs should
be reflected on a Subaward/Consortium budget. This is true even if the money will
not flow through the lead organization. The budget justification can be used to
clarify the flow of funds.
Budget Period:
This field is required.
Identify the specific budget period (for example, 1, 2, 3, 4, 5).
Start Date:
This field is required and may be pre-populated from the SF 424 R&R Form. Enter the
requested/proposed start date of the budget period. For period 1, the start date is typically the
same date as the Proposed Project Start Date on the M.200 - SF 424 (R&R) Form.
End Date:
This field is required. Enter the requested/proposed end date of the budget period.
M - 67

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
A. Senior/Key Person
Who to include in A. Senior/Key Person:
Include the names of senior/key persons at the applicant organization, (or organization leading
the component of a multi-project application), who are involved on the project in a particular
budget period. Include all collaborating investigators and other individuals who meet the
senior/key person definition if they are from the applicant organization.
Consultants designated as senior/key persons in the Senior/Key Person Profile Form can be
included in the "A. Senior/Key Person" section only if they are also employees of the applicant
organization. Otherwise, consultant costs should be included in Consultant Services in Question F
of this form.
Who not to include in A. Senior/Key Person:
Do not list details of collaborators at other institutions here, as they will be provided in the
Subaward Budget for each subaward/consortium organization.
Personnel listed as other significant contributors who are not committing any specific measurable
effort to the project should not be included in the Personnel section (sections "A. Senior/Key
Person" and "B. Other Personnel") since no associated salary and/or fringe benefits can be
requested for their contribution.
Prefix:
Enter the prefix (e.g., Mr., Mrs., Rev.), if applicable, for the name of the senior/key person.
First Name:
This field is required. Enter the first (given) name of the senior/key person.
Middle Name:
Enter the middle name of the senior/key person.
Last Name:
This field is required. Enter the last (family) name of the senior/key person.
Suffix:
Enter the suffix (e.g., Jr., Sr., PhD), if applicable, of the senior/key person.
Base Salary ($):
Enter the annual compensation paid by the employer for the senior/key person. This includes all
activities such as research, teaching, patient care, and other. An applicant organization may choose
to leave this blank; however, NIH or other PHS Agency staff will request this information prior to
award.
Months (Cal./Acad./Sum.):
NIH and other PHS agencies use the concept of “person months” as a metric for determining
percent of effort. For more information about calculating person months, see NIH's information at
Frequently Asked Questions on Person Months.
Identify the number of months the senior/key person will devote to the project in the applicable
box (i.e., calendar, academic, summer).
M - 68

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
Use either calendar months OR a combination of academic and summer months. Measurable
effort is required for every senior/key person entry.
For an explanation of "measurable effort," see the Frequently Asked Questions on Senior/Key
Personnel.
If effort does not change throughout the year, it is OK to use only the calendar months column.
However, you may use both the academic and summer months columns if your institutional
business process requires noting each separately even if effort remains constant. If effort varies
between academic and summer months, leave the calendar months column blank and use only
the academic and summer months columns.
If your institution does not use a 9-month academic year or a 3-month summer period, indicate
your institution’s definition of these in Section L. Budget Justification.
Requested Salary ($):
This field is required. Regardless of the number of months being devoted to the project, indicate
the salary being requested for this budget period for the senior/key person.
Salary limitations. Some PHS grant programs are currently subject to a legislatively imposed
salary limitation. Any adjustment for salary limits will be made at the time of award; therefore,
requested salary should be based on institutional base salary at the time the application is
submitted and not adjusted for any limitation. For guidance on current salary limitations, see the
NIH's Salary Cap Summary or contact your office of sponsored programs.
Graduate student compensation: NIH grants also limit compensation for graduate students.
Compensation includes salary or wages, fringe benefits, and tuition remission. While actual
institutional-based compensation should be requested and justified, this may be adjusted at the
time of the award. For more guidance on this policy, see the NIH Grants Policy Statement, Section
2.3.7.9: Graduate Student Compensation.
Fringe Benefits ($):
Enter the amount of requested fringe benefits, if applicable, for the senior/key person.
Funds Requested ($):
This field is automatically calculated and will reflect the total requested salary and fringe benefits
for the senior/key person.
Project Role:
This field is required. Identify the project role of each senior/key person. Roles should correspond
to the roles included on the M.240 - R&R Senior/Key Person Profile (Expanded) Form. Note that
there must be at least one PD/PI per budget period.
Additional Senior/Key Persons:
If you are requesting funds for more senior/key persons than the form allows, you must include an
attachment listing the additional senior/key person(s) in this “Additional Senior/Key Persons” field.
Use the same format as the budget form and include all the information identified in this section.
Total Funds requested for all persons in the attached file:
If you have attached a file with additional senior/key persons, enter the total funds requested for
everyone listed in the attachment in the “Total Funds requested for all Senior/Key Persons in the
attached file” field.
M - 69

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
Total Senior/Key Persons:
This total will be automatically calculated based on the sum of the “Funds Requested” column and
the “Total Funds requested for all Senior/Key Persons in the attached file” field.
Special Instructions for Joint University and Department of Veterans Affairs (V.A.)
Appointments: Individuals with joint university and V.A. appointments may request the university’s
share of their salary in proportion to the effort devoted to the research project. The individual’s salary
with the university determines the base for computing that request. The signature by the institutional
official on the application certifies that: (1) the individual is applying as part of a joint appointment
specified by a formal Memorandum of Understanding between the university and the V.A.; and (2)
there is no possibility of dual compensation for the same work, or of an actual or apparent conflict of
interest regarding such work. Additional information may be requested by the awarding
components.
B. Other Personnel
Number of Personnel:
For each project role category, identify the number of personnel proposed.
Administrative, Secretarial, and Clerical Support Salaries: In most circumstances, the salaries
of administrative, secretarial, or clerical staff at educational institutions and nonprofit
organizations are included as part of indirect costs (Section H. Indirect Costs). However, examples
of situations where direct charging of administrative or clerical staff salaries may be appropriate
may be found at: 45 CFR 75.403.
Inclusion of such costs may be appropriate only if all of the following conditions are met:
1. Administrative or clerical services are integral to a project or activity;
2. Individuals involved can be specifically identified with the project or activity;
3. Such costs are explicitly included in the budget or have prior written approval of the
federal awarding agency; and
4. The costs are not also recovered as indirect costs.
Requests for direct charging for secretarial/clerical personnel (i.e., administrative and clerical staff)
must be appropriately justified in Section L. Budget Justification. For all individuals classified as
administrative/secretarial/clerical, provide a justification (in the Budget Justification) documenting
how they meet all four conditions. NIH ICs may request additional information for these positions
in order to assess allowability.
Postdoctoral and Graduate Students: For all postdoctoral associates and graduate students not
already named in "Section A. Senior/Key Person," individually list names, roles (e.g., postdoctoral
associates or graduate student), associated months, and requested salary and fringe benefits in
Section L. Budget Justification.
Project Role:
List any additional project role(s) (e.g., engineer, IT professionals, etc.) in the blank(s) provided.
Identify the number of each personnel proposed.
You may have up to six named roles. If you have more than six, you must combine project roles
here and add an explanation about the named roles in Section L. Budget Justification.
M - 70
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
Do not include consultants in this section. Consultants are included below in Section F. Other
Direct Costs.
Months (Cal./Acad./Sum.):
NIH and other PHS agencies use the concept of “person months” as a metric for determining
percent of effort. For more information about calculating person months, see: NIH's Frequently
Asked Questions on Person Months.
Identify the number of months devoted to the project in the applicable box (i.e., calendar,
academic, summer) for each project role category.
Use either calendar months OR a combination of academic and summer months.
If effort does not change throughout the year, it is OK to use only the calendar months column.
However, you may use both academic and summer months columns if your institutional business
process requires noting each separately, even if effort remains constant. If effort varies between
academic and summer months, leave the calendar months column blank and use only the
academic and summer months columns.
If your institution does not use a 9-month academic year or a 3-month summer period, indicate
your institution’s definition of these in Section L. Budget Justification.
Requested Salary ($):
Regardless of the number of months being devoted to the project, indicate only the amount of
salary/wages being requested for this budget period for each project role. The amount entered
should reflect the total amount of funds requested for all personnel within a project role.
Salary limitations: Some PHS grant programs are currently subject to a legislatively imposed
salary limitation. Any adjustment for salary limits will be made at the time of award; therefore,
requested salary should be based on institutional base salary at the time the application is
submitted and not adjusted for any limitation. For guidance on current salary limitations, see the
NIH's Salary Cap Summary or contact your office of sponsored programs.
Graduate student compensation: NIH grants also limit the compensation for graduate students.
Compensation includes salary or wages, fringe benefits, and tuition remission. While actual
institutional-based compensation should be requested and justified, this may be adjusted at the
time of the award. For more guidance on this policy, see the NIH Grants Policy Statement, Section
2.3.7.9: Graduate Student Compensation.
Fringe Benefits ($):
Enter the amount of requested fringe benefits, if applicable, for this project role category. The
amount entered should reflect the total amount of fringe benefits requested for all personnel
within a project role.
Funds Requested ($):
This field will be automatically calculated and will reflect the total requested salary and fringe
benefits for each project role category.
Total Number of Other Personnel:
This total will be automatically calculated based on the Number of Personnel for each project role
category.
M - 71

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
Total Other Personnel:
This total will be automatically calculated based on the sum of the Funds Requested for all Other
Personnel.
Total Salary, Wages and Fringe Benefits (A+B):
This total will be automatically calculated and represents the total Funds Requested for all
Senior/Key persons and all Other Personnel
Special Instructions for Applications Submitted with a Data Management and Sharing Plan:
For applications submitted for due dates on or before October 4, 2023, if a Data
Management and Sharing Plan is required in the proposed application, personnel costs specific to
Data Management and Sharing activities must not be included here but listed as a specific line
item under Section F.8.-17 Other.
For applications submitted for due dates on or after October 5, 2023, DMS costs must be
requested in the appropriate costs category.
C. Equipment Description
The “C. Equipment Description” section is for you to list items and dollar amount for each item
exceeding $5,000 (unless the organization has established lower levels).
Equipment Item:
Equipment is defined as an item of property that has an acquisition cost of $5,000 or more (unless
the organization has established lower levels) and an expected service life of more than one year.
List each item of equipment separately and justify each in Section L. Budget Justification. Allowable
items ordinarily will be limited to research equipment not already available for the conduct of the
work.
Additional Instructions for Multi-project:
Other Components: You are allowed to add up to 100 equipment items in this list.
For additional equipment items, you must list them in the “Additional Equipment”
attachment.
Funds Requested:
This information is required. List the estimated cost of each item, including shipping and any
maintenance costs and agreements.
Additional Equipment:
If you're requesting funds for more equipment than the form allows, you must include an
attachment listing the additional equipment items in this “Additional Equipment” field. Enter the
information in a separate file and attach it as a PDF. List each additional item and the funds
requested for each individual item. The dollar amount for each item should exceed $5,000 (unless
the organization has established lower levels).
Total funds requested for all equipment listed in the attached file:
If you have attached a file with additional equipment, enter the total funds requested for all the
equipment listed in the attachment.
M - 72

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
Total Equipment:
This total will be automatically calculated based on the sum of the “Funds Requested” column and
the “Total funds requested for all equipment listed in the attached file” field.
D. Travel
1. Domestic Travel Costs (Incl. Canada, Mexico, and U.S. Possessions):
Enter the total funds requested for domestic travel. Domestic travel includes destinations in the
U.S., Canada, Mexico, and U.S. possessions. In Section L. Budget Justification, include the purpose,
destination, dates of travel (if known), and the number of individuals for each trip. If the dates of
travel are not known, specify the estimated length of trip (e.g., 3 days).
2. Foreign Travel Costs:
Identify the total funds requested for foreign travel. Foreign travel includes any destination outside
of the U.S., Canada, Mexico, or U.S. possessions. In Section L. Budget Justification, include the
purpose, destination, dates of travel (if known), and the number of individuals for each trip. If the
dates of travel are not known, specify the estimated length of trip (e.g., 3 days).
Total Travel Cost:
This total will be automatically calculated based on the sum of the Domestic and Foreign Funds
Requested fields.
E. Participant/Trainee Support Costs
Unless specifically stated otherwise in a FOA, NIH and other PHS agencies applicants should skip
Section E. Participant/Trainee Support Costs. Note: Tuition remission for graduate students should
be included in Section F. Other Direct Costs when applicable.
1. Tuition/Fees/Health Insurance:
List the total funds requested for Participant/Trainee Tuition/Fees/Health Insurance.
2. Stipends:
List the total funds requested for Participant/Trainee stipends.
3. Travel:
List the total funds requested for Participant/Trainee travel.
4. Subsistence:
List the total funds requested for Participant/Trainee subsistence.
5. Other:
Describe any other Participant/Trainee support costs and list the total funds requested for all other
Participant/Trainee costs described.
Number of Participants/Trainees:
List the total number of proposed Participants/Trainees. Value cannot be greater than 999.
Total Participant/Trainee Support Costs:
This field is required if any data has been entered in “Section E. Participant/Trainee Support Costs.”
This total will be automatically calculated based on the sum of the Funds Requested column in
M - 73

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
"Section E. Participant/Trainee Support Costs."
F. Other Direct Costs
1. Materials and Supplies:
List the total funds requested for materials and supplies. In Section L. Budget Justification, indicate
general categories such as glassware, chemicals, animal costs, etc., including an amount for each
category. Categories with amounts less than $1,000 are not required to be itemized.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If costs for
human fetal tissue obtained from elective abortions (HFT) as defined in the NIHGrants Policy
Statement are included in the proposed budget, they must not be included here but listed as a
specific line item under Section F.8-17 Other.
2. Publication Costs:
List the total funds requested for publication costs. The proposal budget may request funds for the
costs of documenting, preparing, publishing, or otherwise making available to others, the findings
and products of the work conducted under the award. Include supporting information in Section L.
Budget Justification.
3. Consultant Services:
List the total funds requested for all consultant services. Identify the following items in Section L.
Budget Justification, as applicable:
l
each consultant, the services he/she will perform, total number of days, travel costs, and
the total estimated costs;
l
the names and organizational affiliations of all consultants, other than those involved in
consortium/contractual arrangements;
l
consulting physicians in connection with patient care; and
l
persons who are confirmed to serve on external monitoring boards or advisory
committees to the project. Describe the services to be performed.
4. Automatic Data Processing (ADP)/Computer Services:
List the total funds requested for ADP/computer services. The cost of computer services, including
computer-based retrieval of scientific, technical, and education information may be requested. In
Section L. Budget Justification, include the established computer service rates at the proposing
organization, if applicable.
5. Subawards/Consortium/Contractual Costs:
List the total funds requested for:
1. all subaward/consortium organization(s) proposed for the project and
2. any other contractual costs proposed for the project.
This line item should include both direct and indirect costs for all subaward/consortium
organizations.
Contractual costs for support services, such as laboratory testing of biological materials, clinical
services, or data processing, are occasionally sufficiently high to warrant a categorical breakdown
M - 74
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
of costs. When this is the case, provide detailed information as part of Section L. Budget
Justification.
NIH policy provides for exclusion of consortium/contractual F&A costs when determining if an
applicant is in compliance with a direct cost limitation. However, you must include the full cost of
consortium/subawards in this field. See the NIH Grants Policy Statement, Section 2.3.7.1:
Applications that Include Consortium/Contractual F&A Costs for policy related to the exclusion of
consortium/subaward amounts in determining whether an applicant is in compliance with a direct
cost limitation.
6. Equipment or Facility Rental/User Fees:
List the total funds requested for equipment or facility rental/user fees. In Section L. Budget
Justification, identify and justify each rental user fee.
7. Alterations and Renovations:
List the total funds requested for alterations and renovations (A&R). In Section L. Budget
Justification, itemize by category and justify the costs of alterations and renovations, including
repairs, painting, and removal or installation of partitions, shielding, or air conditioning. Where
applicable, provide the square footage and costs.
Under certain circumstances the public policy requirements that apply to construction activities
may also apply to A&R activities. Refer to the NIH Grants Policy Statement, Section 10.10:
Construction Grants – Public Policy Requirements and Objectives for more information.
Special Instructions for Foreign Organizations (Non-domestic [non-U.S.] Entities): Minor
A&R costs (≤$500,000) are allowable on applications from foreign organizations and domestic
institutions with foreign components. When requesting minor A&R costs under this policy,
please provide detailed information on the planned A&R in the budget justification.
8-17 Other:
Add descriptions for any “other” direct costs not requested above. Use Section L. Budget
Justification to further itemize and justify.
List funds requested for each of the items in lines "8-17 Other.” Use lines 8-17 for costs such as
patient care costs, tuition remission and SBIR/STTR "Technical Assistance"(TABA) costs. If
requesting patient care costs, request inpatient and outpatient costs separately.
Lines "8-17 Other" may also be used to request direct costs related to the use of single
Institutional Review Board (sIRB)for multi-site human subjects research.
For more information on charging direct and indirect costs for single IRBactivities, see the
Scenarios to Illustrate the Use of Direct and Indirect Costs for Single IRBReview under the
NIHPolicy on the Use of a Single IRBfor Multi-Site Research.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) is included in the proposed application, regardless of whether costs will be incurred, it
must be noted as a single line item here. The line item must be titled “Human Fetal Tissue Costs”
(without quotation marks, but following exact phrase and spacing). The line item must only be
used for HFT costs and cannot include or be combined with any “Other” costs. If no cost will be
incurred (e.g. if HFT will be donated), enter “0” in the “Funds Requested” column. Details regarding
HFT must be specified in the Budget Justification attachment (L), pursuant to the instructions.
M - 75

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
Applications proposing HFT that do not address these requirements will be administratively
withdrawn. For further information on HFTpolicy refer to the NIHGrants Policy Statement, Section
2.3.7.11 Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research
and Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
Special Instructions for Applications Submitted with a Data Management and Sharing
(DMS) Plan:
For applications submitted on or before October 4, 2023, NIH recognizes that making data
accessible and reusable for other researchers may incur costs. If a Data Management and Sharing
Plan is required in the proposed application (see instructions for the “Other Plan(s)” attachment on
the PHS 398 Research Plan Form and the PHS 398 Career Development Award Supplemental Form,
as applicable), costs to support these activities, including personnel costs (e.g., personnel who will
be curating data for the project) must be noted as a single line item. The line item must be titled
"Data Management and Sharing Costs" (without quotation marks, but following exact phrase and
spacing). The line item must only be used for Data Management and Sharing costs and cannot
include or be combined with any "Other" costs. If no cost will be incurred, enter "0" in the "Funds
Requested" column. Details regarding Data Management and Sharing costs must be specified in
the Budget Justification attachment (L), pursuant to the instructions.
For applications submitted for due dates on or after October 5, 2023, NIH recognizes that
making data accessible and reusable for other researchers may incur costs. If a Data Management
and Sharing Plan is required in the proposed application (see instructions for the “Other Plan(s)”
attachment on the PHS 398 Research Plan Form and the PHS 398 Career Development Award
Supplemental Form, as applicable), costs to support these activities, may be requested in the
appropriate cost category. Details regarding Data Management and Sharing costs must be
specified in the Budget Justification attachment (L), pursuant to the instructions.
Allowable and Unallowable Costs: Allowable costs submitted in budget requests must be
incurred during the performance period, even for scientific data and metadata preserved and
shared beyond the award period. Budget requests must NOT include: Infrastructure costs that are
included in institutional overhead (for instance, NIH Grants Policy Statement Section 7.3 Facilities
and Administrative costs); costs associated with the routine conduct of research, including costs
associated with collecting or gaining access to research data; or costs that are double charged or
inconsistently charged as both direct and indirect costs. For more information, see Budgeting for
Data Management & Sharing on the NIH Scientific Data Sharing website and additional details to
help Develop Your Budget.
Additional Instructions for Multi-project:
Other Components, Special Instructions for Patient Care Costs: If inpatient
and/or outpatient costs are requested, provide the names of any hospitals and/or
clinics and the amounts requested for each in the Budget Justification.
State whether each hospital or clinic has a currently effective HHS-negotiated
research patient care rate agreement and, if not, what basis is used for calculating
costs. If an applicant does not have a HHS-negotiated rate, the PHS awarding
component can approve a provisional rate. Indicate, in detail, the basis for
estimating costs in this category, including the number of patient days, estimated
cost per day, and cost per test or treatment. If multiple sites are to be used, provide
detailed information by site.
M - 76

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
Include information regarding projected patient accrual for the project/budget
periods and relate this information to the budget request for patient care costs. If
patient accrual is anticipated to be lower at the start or during the course of the
project, plan budget(s) accordingly.
Provide specific information regarding anticipated sources of Other Support for
patient care costs, e.g., third party recovery or pharmaceutical companies. Include
any potential or expected utilization of the Clinical and Translational Science Awards
(CTSA) program.
Special Instructions for Applications Submitted with a Data Management
and Sharing (DMS) Plan
Other Components: Include budget information for Data Management and Sharing
Costs within the applicable component(s). Do not include a “Data Management and
Sharing Plan” attachment within the components. Any component-specific
information should be described within the overall “Data Management and Sharing
Plan” attachment provided within the PHS 398 Research Plan Form in the Overall
Component.
Total Other Direct Costs:
This total will be automatically calculated based on the sum of the Funds Requested column in
"Section F. Other Direct Cost."
G. Direct Costs
This total will be automatically calculated based on the sum of the Total funds requested for all
direct costs (sections A-F).
H. Indirect Costs
Indirect costs (Facilities & Administrative [F&A] costs) are defined as costs that are incurred by a
grantee for common or joint objectives and that, therefore, cannot be identified specifically with a
particular project or program. See the NIH Glossary's definition of Indirect Costs.
For more information:
You are encouraged to visit the following Division of Financial Advisory Services (DFAS) Websites
or call DFAS staff at 301-496-2444 for guidance: Main DFAS website, DFAS Frequently Asked
Questions. The following website has a listing of unallowable and unallocable costs and the related
Federal Acquisition Regulation (FAR) citation for each: NIH Office of Management's
Unallowable/Unallocable Costs.
Refer to the NIH Grants Policy Statement, Section 7.4: Reimbursement of Facilities and
Administrative Costs for more information.
Special Instructions for Foreign Organizations (Non-domestic [non-U.S.] Entities): Foreign
institutions and international organizations may request funds for limited F&A costs (8% of modified
total direct costs less equipment) to support the costs of compliance with HHS and NIH requirements
including, but not limited to, those related to the protection of human subjects, animal welfare,
invention reporting, financial conflict of interest, and research misconduct. Foreign organizations
M - 77

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
may not include any charge-back of customs and import fees, such as consular fees, customs surtax,
value-added taxes (VAT), and other related charges.
Indirect Cost Type:
Enter the type of indirect cost (e.g., Salary & Wages, Modified Total Direct Costs, etc.) and whether
the cost is off-site. If more than one rate or base is involved for a given type of indirect cost, then
list them as separate entries. If you do not have a current indirect (F&A) rate(s) approved by a
federal agency, indicate “None--will negotiate” and include information for a proposed rate. Use
Section L. Budget Justification if additional space is needed.
Indirect Cost Rate (%):
Enter the most recent indirect cost rate(s) established with the cognizant federal office, or in the
case of for-profit organizations, the rate(s) established with the appropriate agency. If you have a
cognizant/oversight agency and are selected for an award, you must submit your indirect rate
proposal to the NIH awarding IC or to the PHS awarding office for approval. If you do not have a
cognizant/oversight agency, contact the awarding agency. This field should be entered using a
rate such as “55.5.”
Indirect Cost Base ($):
Enter the amount of the base for each indirect cost type.
Funds Requested ($):
Enter the funds requested for each indirect cost type.
Total Indirect Costs:
This total will be automatically calculated from the “Funds Requested” column in "Section H.
Indirect Cost."
Cognizant Federal Agency:
Enter the name of the cognizant Federal Agency and the name and phone number of the
individual responsible for negotiating your rate (your point of contact). If no cognizant agency is
known, enter “None.”
I. Total Direct and Indirect Costs
This total will be automatically populated from the sum of Total Direct Costs (from Section G.
Direct Cost) and the Total Indirect Costs (from Section H. Indirect Costs).
J. Fee
Do not include a fee in your budget, unless the FOA specifically allows inclusion of a “fee.” If a fee
is allowable, enter the requested fee.
K. Total Costs and Fee
This total will be automatically calculated from the sum of Total Direct Costs and Fee (from
sections “I. Total Direct and Indirect Costs” and “J. Fee”).
M - 78
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
L. Budget Justification
The “Budget Justification” attachment is required. Attach only one file.
Use the Budget Justification to provide the additional information requested in each budget
category identified above and any other information the applicant wishes to submit to support the
budget request. If you have a quote(s), you may include it here (information in the quote may be
not used to supplement information provided in page-limited sections of the application, such as
the Research Strategy). The following budget categories must be justified, where applicable:
equipment, travel, participant/trainee support, and other direct cost categories.
In addition to the justifications described in the above sections, also include a justification for any
significant increases or decreases from the initial budget period. Justify budgets with more than a
standard escalation from the initial to the future year(s) of support.
Also use the Budget Justification to explain any exclusions applied to the F&A base calculation.
If your application includes a subaward/consortium budget, a separate Budget Justification must
be submitted. See M.310 - R&R Subaward Budget Attachment(s) Form.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) is included in the proposed application include a detailed justification including the
quantity, type(s), and source(s) of the HFT, including the stage of fetal development. This
information must be included if costs for the HFT are assigned to the grant or if the HFT is
acquired under the grant at no costs. The HFT justification must be clearly labeled in the budget
justification attachment.
Special Instructions for Applications Submitted with a Data Management and Sharing
(DMS) Plan:
If a Data Management and Sharing Plan is required in the proposed application (see instructions
for the “Other Plan(s)” attachment on the PHS 398 Research Plan Form and the PHS 398 Career
Development Award Supplemental Form, as applicable), include a brief justification of the
proposed activities that will incur costs. The Data Management and Sharing justification must be
clearly labeled as “Data Management and Sharing Justification” within the budget justification
attachment followed by the estimated dollar amount (total direct costs). Provide a brief summary
of type and amount of scientific data to be preserved and shared and the name of the established
repository(ies) where they will be preserved and shared. Indicate general cost categories such as
curating data and developing supporting documentation, local data management activities,
preserving and sharing data through established repositories, etc., including an amount for each
category and a brief explanation. Specify in the justification if no costs will be incurred for Data
Management and Sharing, if applicable. The recommended length of the justification should be no
more than half a page. For more information, see Budgeting for Data Management & Sharing on
the NIH Scientific Data Sharing website and additional details to help Develop Your Budget.
Research & Related Budget - Cumulative Budget
All values on this form are automatically calculated, and the fields are pre-populated. They present
the summations of the amounts you entered previously, under Sections A through K, for each of
the individual budget periods. Therefore, no data entry is allowed or required to complete this
“Cumulative Budget” section.
M - 79

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.300 - R&R Budget Form
If any of the amounts displayed on this form appear to be incorrect, you may correct it by
adjusting one or more of the values that contribute to that total. To make any such corrections,
you will need to revisit the appropriate budget period form(s).
M - 80

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.310 - R&R Subaward Budget Attachment(s) Form
M.310 - R&R Subaward Budget
Attachment(s) Form
The R&R Subaward Budget Attachment(s) Form is used
for applications with a subaward or consortium.
This form is required only when the prime grantee is
submitting an R&R Budget Form and has
subaward/consortium budgets.
Applicants using the Modular Budget Form should see
M.320 - Modular Budget Form for instructions
concerning information on consortium budgets.
View larger image
Who should use the R&R Subaward Budget Attachment(s) Form?
The R&R Subaward Budget Attachment(s) Form is required if you have a subaward/consortium and are
using the M.300 - R&R Budget Form.
Do not use this form if you are using the PHS Modular Budget Form or if you do not have a
subaward/consortium.
Each consortium grantee organization that performs a substantive portion of the project must complete
an R&R Subaward Budget Attachment, including the Budget Justification section.
Consortium/Contractual F&A Costs:
NIH policy provides for the exclusion of consortium/contractual F&A costs when determining if an
applicant is in compliance with a direct cost limitation. However, you must include the full cost of
subaward/consortium in the Subawards/Consortium Costs field (M.300 - R&R Budget Form, Section F.
Other Direct Costs, Question 5). If a subaward/consortium is not performing a substantive portion of
the project, they do not need to complete an R&R Subaward Budget Form; however, their costs must be
included in the prime grantee’s R&R Budget Form. All F&A costs count toward the direct cost limit.
Refer to the NIH Grants Policy Statement, Section 2.3.7.1: Applications That Include
Consortium/Contractual F&A Costs for policy related to the exclusion of consortium/subaward amounts
in determining whether an applicant is in compliance with a direct cost limitation.
Applicants should document how their budget falls below the direct cost limit in their Budget
Justification on the R&R Subaward Budget Form.
Note on Project Roles for Consortium Lead Investigators:
It is appropriate and expected that someone may serve as the consortium lead investigator responsible
for ensuring proper conduct of the project or program at each subaward or consortium site.
M - 81

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.310 - R&R Subaward Budget Attachment(s) Form
Unless you are submitting your application under the multiple PD/PI policy, consortium lead
investigators are NOT considered PD/PIs for the “Project Role” field. This individual should be assigned
some other project role on the M.300 - R&R Budget Form and in the M.240 – R&R Senior/Key Person
Profile (Expanded) Form. However, the project role of “PD/PI” should be used for a consortium lead
investigator if they also serve as PD/PI for the entire application under the multiple PD/PI policy.
Using the R&R Subaward Budget Attachment(s) Form:
The location of the R&R Subaward Budget Attachment(s) Form may vary with the type of submission
(e.g., under an “Optional Forms” tab).
The steps needed to include a subaward budget in your application vary by submission method. If
submitting using the Grants.gov Workspace, the prime applicant can extract a copy of the R&R Budget
Form from the R&R Subaward Budget Attachment(s) Form and send the extracted file to the
consortium for completion. After the consortium completes the R&R Budget Form, following the
instructions here and in M.300 – R&R Budget Form, the prime grantee must then upload the R&R
Budget Form to the R&R Subaward Budget Attachment(s) Form.
For all submission methods, the R&R Budget Form with a "Budget Type" of Subaward/Consortium is
used to collect subaward budget data. However, ASSIST and other system-to-system solutions may
present a different interface than the R&R Subaward Budget Attachment Form shown here.
This form accommodates a set number of separate subaward budgets. If you need to add more
subaward budgets than the form allows, include the remaining budgets as part of Budget Justification
in M.300 – R&R Budget Form.
Regardless of how many subaward budgets you include, the sum of all subaward budgets (those
attached within the R&R Subaward Budget Attachment(s) Form and those provided as part of the
project budget’s Budget Justification), must be included in M.300 - R&R Budget Form, Section F. Other
Direct Costs, Question 5. Subawards/Consortium/Contractual Costs of the project budget.
Format:
All attachments, including all Subaward Budget Forms and Budget Justifications, must be PDF files. The
R&R Budget Forms are already PDFs when extracted. Do not alter the format. Use of hyperlinks and
URLs in this section is not allowed unless specified in the funding opportunity announcement.
Content:
On this R&R Subaward Budget Attachment(s) Form, you will attach the R&R Subaward Budget files for
your application. Each consortium should complete the Subaward Budget(s) in accordance with the
M.300 - R&R Budget Form instructions.
Submitting Subaward Budgets that are not Active for all Periods of the Prime Grant:
The R&R Budget Forms do not allow for “empty” budget periods.
Subaward/consortiums organizations should complete all budget periods in the R&R Subaward Budget
Form for their subaward budgets, aligning the budget period numbers, start dates, and end dates with
the budget periods of the prime grant.
Example: The prime fills out an R&R Budget Form with the following periods:
l
period 1 - Jan 1, 2017 – Dec 31, 2017
l
period 2 - Jan 1, 2018 – Dec 31, 2018
l
period 3 - Jan 1, 2019 – Dec 31, 2019
M - 82

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.310 - R&R Subaward Budget Attachment(s) Form
l
period 4 - Jan 1, 2020 – Dec 31, 2020
l
period 5 - Jan 1, 2021 – Dec 31, 2021
The budget period numbers and dates should be the same in all the R&R Subaward Budget Forms
included in the R&R Subaward Budget Attachment(s) Form.
The R&R Subaward Budget Forms include several required fields which must be completed (even for
inactive periods) in order to successfully submit the application. Provide the following information for
inactive budget periods in subaward/consortium budgets:
l
Unique Entity Identifier
l
Budget Type = Subaward/Consortium
l
Budget Period Start/End Dates (align with budget periods and dates of the prime budget)
l
In Question "A: Senior/Key Person," provide a single entry including the following:
o
PD/PI or subaward lead First and Last names
o
Project Role (may default to PD/PI; can be adjusted as needed)
o
Calendar Months = .01 (smallest amount effort allowed in the field)
o
Requested Salary = $0
o
Fringe Benefits = $0
l
Explanation of the inactive budget periods in the Budget Justification of the
subaward/consortium's R&R Subaward Budget Form
M - 83

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.330 - PHS 398 Training Budget Form
M.330 - PHS 398 Training Budget Form
The PHS 398 Training Budget Form is used only for
Training applications (e.g., T15, T32, T34, T35, T36, T90)
and Multi-project applications with a training
component.
The PHS 398 Training Budget Form is not applicable for
the K12, T37, D43, D71, or U2R activity codes. Applicants
to these activity codes should follow the instructions for
the R&R Budget Form and the instructions in the FOA (if
applicable).
For current stipend levels and allowable costs, refer to
the relevant FOA, NIH’s Research Training &Career
Development website, or consult the PHS awarding
component.
View larger image
Quick Links
Introductory Fields
A. Stipends, Tuition/Fees
B. Other Direct Costs
C. Total Direct Costs Requested (A+B)
D. Indirect (F&A) Costs
E. Total Direct and Indirect (F&A) Costs Requested (C+D)
F. Budget Justification
PHS 398 Training Budget, Cumulative Budget
Who should use the PHS 398 Training Budget Form?
Use this form if you will be submitting certain types of Training Applications (e.g., T15, T32, T34, T35,
T36, or T90), regardless of the amount of the requested budget.
If you are requesting a budget with $500,000 or more in direct costs for any budget period, contact the
awarding component to determine whether you must obtain prior approval before submitting the
application. For more information on applications that request $500,000 or more in direct costs, see the
NIH Grants Policy Statement, Section 2.3.7.2: Acceptance for Review of Unsolicited Applications
Requesting $500,000 or More in Direct Costs.
Certain types of Training Applications, such as K12, T37, D43, D71, and U2R, do not use the PHS 398
Training Budget Form. These applications use the R&R Budget Form.
Note on Subawards/Consortiums: If you have a subaward/consortium, you must use the PHS 398
Training Subaward Budget Attachment(s) Form in conjunction with the PHS 398 Training Budget Form.
M - 84

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.330 - PHS 398 Training Budget Form
The prime must extract the PHS 398 Training Subaward Budgets from the PHS 398 Training Subaward
Budget Attachment(s) Form and send the extracted file to the subaward/consortium. The consortium
should complete the PHS 398 Training Subaward Budget, following the instructions here and in M.340 –
PHS 398 Training Subaward Budget Attachment(s) Form.
Using the PHS 398 Training Budget Form:
You must complete a separate training budget for each budget period requested. The form will
generate a cumulative budget for the total project period. If no funds are requested for a required field,
leave the field blank.
You must round to the nearest whole dollar amount in all dollar fields.
Introductory Fields
Unique Entity Identifier (UEI):
This field is required. This field may be pre-populated from the SF 424 (R&R) Form and should
reflect the UEI of the applicant organization.
Budget Type:
This field is required. Check the appropriate box for your budget type, following these guidelines.
Project: The budget being requested is for the primary applicant organization.
Subaward/Consortium: The budget being requested is for the subaward/consortium
organization(s). Note: Separate budgets are required only for subaward/consortium organizations
that perform a substantive portion of the project.
If you are preparing an application that includes a subaward/consortium, in addition to
completing this form, also see M.340 – PHS 398 Training Subaward Budget Attachment(s) Form.
Organization Name:
This field may be pre-populated from the M.200 - SF 424 (R&R) Form.
Start Date:
This field is required and may be pre-populated from the M.200 - SF 424 (R&R) Form. Enter the
requested/proposed start date of the budget period. For period 1, the start date is typically the
same as the Proposed Project Start Date on the SF 424 (R&R) Form.
End Date:
This field is required. Enter the requested/proposed end date of the budget period.
A. Stipends, Tuition/Fees
Number of Trainees
Enter the number of trainees for each category (undergraduate, predoctoral, postdoctoral, and
other), distinguishing between full-time training positions (i.e., a full year of training) and short
term trainees.
Note that some programs do not allow all categories of trainees (e.g., undergraduates are not
eligible for T32 applications). Refer to your FOA regarding the eligible types of trainees for your
specific application.
M - 85

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.330 - PHS 398 Training Budget Form
l
For undergraduate trainees: list separately the number that will be at the First-Year/Sophomore
stipend level and the number that will be at the Junior/Senior stipend level in the boxes
provided.
l
For predoctoral trainees: list separately the number that will be pursuing single degrees and
the number that will be pursuing dual degrees in the boxes provided. The "Total Predoctoral"
fields will be automatically calculated.
l
For postdoctoral trainees: list separately the number that are non-degree seeking and the
number that are degree seeking in the boxes provided. If a category (non-degree seeking or
degree seeking) contains various stipend levels (e.g., for varying levels of postdoctoral
experience or for varying appointment periods), itemize the number of postdoctoral trainees
by stipend level in the boxes provided. The "Total Postdoctoral" fields will be automatically
calculated.
Stipends Requested ($)
Enter the total stipend amount requested for each trainee type.
For current stipend levels and allowable costs, refer to the FOA or consult the PHS awarding
component. For more information, see the NIH’s Research Training and Career Development
website.
The “Total Stipends Requested” field will be automatically calculated.
Tuition/Fees Requested ($)
Enter the total tuition/fees requested for each trainee type.
See the NIH Grants Policy Statement, Section 11.3.8: Allowable and Unallowable Costs for NIH
policy regarding payment of tuition and fees.
Tuition at the postdoctoral level is limited to that required for specified courses that are to be
described in Section F. Budget Justification and may depend on whether the program supports
postdoctoral individuals in formal degree-granting training.
The “Total Tuition/Fees Requested” field will be automatically calculated.
You should request full needs for tuition and fees. The awarding component will determine the
amount of tuition and fees to be provided according to the policies current at the time of award.
The formula currently in effect will be applied by the NIH awarding component at the time an
award is calculated. Do not include health insurance in the tuition/fees fields.
Total Stipends + Tuition/Fees Requested
This total will be automatically calculated.
B. Other Direct Costs
Enter the total funds requested for Trainee Travel and Training Related Expenses (TRE). If
applicable, enter the Total Direct Costs from the R&R Budget Form and Consortium Training Costs.
Trainee Travel
Enter the total funds requested for trainee travel in the "Trainee Travel" field.
Some NIH awarding components provide a pre-determined amount for travel for each full time
trainee. Refer to the FOA and/or contact the awarding component to determine the amount
M - 86

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.330 - PHS 398 Training Budget Form
provided for travel and enter it here. If the awarding component does not provide a pre-
determined amount, enter the requested amount here and provide an explanation in Section F.
Budget Justification, stating the purpose of any travel, giving the number of trips involved, the
destinations, and the number of trainees for whom funds are requested. PHS policy requires coach
class air travel be used. Justify any foreign travel in detail, describing its importance to the training
experience.
Training Related Expenses
Enter the total funds requested for TRE. You must base your requested amount on the number of
trainees at the predetermined rate.
Funds to defray other costs of training, such as health insurance, staff salaries, consultant costs,
equipment, research supplies, staff travel, etc., are requested as a lump sum based on the amounts
specified in the FOA and in the NIH Grants Policy Statement, Section 11.3.8.4: Training-Related
Expenses for each predoctoral and postdoctoral trainee.
Health insurance may be covered by TRE only to the extent that the same health insurance fees are
charged to non-federally-supported students and postdoctoral fellows.
TRE will be awarded as a lump sum. No further itemization or explanation is required in Section F.
Budget Justification.
The awarding component will apply the TRE level established for institutional programs for the
relevant fiscal year at the time of award.
Total Direct Costs from R&R Budget Form (if applicable)
Certain FOAs allow funds to cover direct costs for items other than those specified above. Use the
R&R Budget Form to submit those costs. The Total Direct Costs from the R&R Budget Form (M.300
- R&RBudget Form, Section G. Direct Costs) should be inserted here. This line should not include
any indirect costs.
Additional Instructions for Multi-project:
Skip the “Total Direct Costs from R&R Budget Form” field, as Kirschstein-NRSA
Training components do not include the R&R Budget Form.
Consortium Training Costs (if applicable)
If training occurs at more than one institution and there is a transfer of funds between
organizations, you must complete the M.340 - PHS 398 Training Subaward Budget Attachment(s)
Form. Total the direct costs from the Training Subaward Budget Attachment Forms and insert the
total here. The applicant institution is responsible and accountable for any arrangements,
expenditures, and submission of all required application forms when more than one institution is
involved in the research training program.
Total Other Direct Costs Requested
This total will be automatically calculated based on the sum of the funds requested in "B. Other
Direct Costs."
C. Total Direct Costs Requested (A+B)
This total will be automatically calculated based on the sum of the funds requested in both "A.
Stipends, Tuition/Fees" and "B. Other Direct Costs."
M - 87

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.330 - PHS 398 Training Budget Form
D. Indirect (F&A) Costs
Indirect costs (Facilities & Administrative [F&A] costs) are defined as costs that are incurred by a
grantee for common or joint objectives and that, therefore, cannot be identified specifically with a
particular project or program. See the NIHGlossary's definition of Indirect Costs.
Equipment and consortium costs are also excluded from the F&A costs on those training grants
where TRE are not calculated and awarded on a lump-sum basis, such as the Maximizing Access to
Research Careers Program (MARC).
State and local government agencies will receive the full F&A cost rate.
For more information:
You are encouraged to visit the following Division of Financial Advisory Services (DFAS) Websites
or call DFAS staff at 301-496-2444 for guidance: Main DFAS website, DFAS Frequently Asked
Questions. The following website has a listing of unallowable and unallocable costs and the related
Federal Acquisition Regulation (FAR) citation for each: NIH Office of Management's
Unallowable/Unallocable Cost.
Indirect (F&A) Type:
Enter “F&A.”
Indirect (F&A) Rate (%):
Enter “8.”
Facilities and Administrative (F&A) costs under Institutional Kirschstein-NRSA awards, other than
those issued to U.S., state, or local government agencies, will be awarded at 8%.
State and local government agencies should enter their full F&A cost rate.
Indirect (F&A) Base ($):
Enter the sum of the stipends and the Total Other Direct Costs requested, regardless of whether
those direct costs were listed on the PHS 398 Training Budget Form or on the R&R Budget Form.
Indirect costs are not paid on Tuition/Fees, equipment, or sub-grants and contracts in excess of
$25,000.
Funds Requested ($):
Enter the product of Indirect (F&A) Rate and the Indirect (F&A) Base. Refer to the NIH Grants Policy
Statement, Section 7.4: Reimbursement of Facilities and Administrative Costs for more
information.
E. Total Direct and Indirect (F&A) Costs Requested (C+D)
This total will be automatically calculated based on the sum of the "C. Total Direct Costs
Requested" and "D. Total Indirect (F&A) Costs Requested" fields.
F. Budget Justification
A Budget Justification attachment is required.
Attach one file for the entire project period. Hyperlinks and URLs are not allowed unless specified
in the funding opportunity announcement.
M - 88
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.330 - PHS 398 Training Budget Form
Explain in detail the composition of any of the above costs, as necessary, according to the
guidelines listed here:
l
Itemize tuition and individual fees. If tuition varies, (e.g., in-state, out-of-state, student status)
list these separately. Do not include health insurance in the tuition and fees category.
l
If tuition is requested for postdoctoral trainees, the specific courses or formal degree-granting
program must be described.
l
If the awarding component does not provide a pre-determined amount for travel for each full
time trainee, explain the requested amount and describe the purpose of any travel, indicating
the expected number of trips involved, the likely destinations, and the number of trainees for
whom funds are requested, bearing in mind that PHS policy requires coach class air travel be
used.
l
Any foreign travel must be justified in detail. Describe its importance to the training experience
and how those opportunities differ from and complement those offered by the grantee
institution. Also describe the relationship of the proposed off-site training experience to the
career stage of the grantee.
l
Justify the number of training slots (e.g., predoctoral and/or postdoctoral) requested. For
postdoctoral training slots, justify the stipend levels requested.
Note for Applicants Using both the PHS 398 Training Budget Form and the R&R Budget
Form: Generally, the Budget Justification included in the PHS 398 Training Budget Form should
reflect only funds requested on the PHS 398 Training Budget Form. When the R&R Budget Form is
also used, two separate Budget Justifications are required, each covering the costs requested in
the respective Budget Form.
PHS 398 Training Budget, Cumulative Budget
All values on this form are automatically calculated, and the fields are pre-populated. They present
the summations of the amounts you entered previously for each of the individual budget periods.
Therefore, no data entry is allowed or required to complete the “Cumulative Budget” section.
If any of the amounts displayed on this form appear to be incorrect, you may correct it by
adjusting one or more of the values that contribute to that total. To make any such corrections,
you will need to revisit the appropriate budget period form(s).
M - 89

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.340 - PHS 398 Training Subaward Budget Attachment(s) Form
M.340 - PHS 398 Training Subaward
Budget Attachment(s) Form
The PHS 398 Training Subaward Budget Attachment(s)
Form is used for applications with a subaward or
consortium.
This form is required only when the prime grantee is
submitting a PHS 398 Training Budget Form and has
subaward/consortium budgets.
Applicants using the R&R Budget Form should see M.300
- R&R Budget Form.
View larger image
Who should use the PHS 398 Training Subaward Budget
Attachment(s) Form?
The PHS 398 Training Subaward Budget Attachment(s) Form is required if you have a
subaward/consortium and are using the PHS 398 Training Budget Form.
Do not use this form if you do not have a subaward/consortium.
Each subaward/consortium that performs a substantive portion of the project must complete a Training
Subaward Budget, including the Budget Justification section. For most programs, this is not common
but is usually encountered when a portion of the training program takes place at a site other than the
applicant organization via a collaborative or consortium arrangement. In such situations, the applicant
organization is responsible and accountable for acceptable training arrangements, expenditure of
funds, and submission of all required forms.
Consortium/Contractual F&A Costs:
NIH policy provides for the exclusion of consortium/contractual F&A costs when determining if an
applicant is in compliance with a direct cost limitation. However, you must include the full cost of
consortium/subawards in the Subawards/Consortium Costs field. If a subaward/consortium is not
performing a substantive portion of the project, they do not need to complete a Training Subaward
Budget; however, their costs must be included in the prime grantee’s Training Budget Form. All F&A
costs count toward the direct cost limit.
See the NIH Grants Policy Statement, Section 2.3.7.1: Applications That Include Consortium/Contractual
F&A Costs for policy related to the exclusion of consortium/subaward amounts in determining whether
an applicant is in compliance with a direct cost limitation.
Applicants should document how their budget falls below the direct cost limit in the Budget
Justification of the Training Subaward Budget.
M - 90

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.340 - PHS 398 Training Subaward Budget Attachment(s) Form
Note on Project Roles for Consortium Lead Investigators:
It is appropriate and expected that someone may serve as the consortium lead investigator responsible
for ensuring proper conduct of the project or program at each subaward or consortium site.
Unless you are submitting your application under the multiple PD/PI policy, consortium lead
investigators are NOT considered PD/PIs for the “Project Role” field. This individual should be assigned
some other project role on the PHS398 Training Budget Form and in the M.240 – R&R Senior/Key
Person Profile (Expanded) Form. However, the project role of “PD/PI” should be used for a consortium
lead investigator if they also serve as PD/PI for the entire application under the multiple PD/PI policy.
Using the PHS 398 Training Subaward Budget Attachment(s) Form:
The location of the PHS 398 Training Subaward Budget Attachment(s) Form may vary with the type of
submission (e.g., under an “Optional Forms” tab).
The steps needed to include a subaward budget in your application vary by submission method. If
submitting using the Grants.gov Workspace, the prime applicant can extract a copy of the Training
Subaward Budget Form from the Training Subaward Budget Attachment(s) Form and send the
extracted file to the consortium for completion. After the consortium completes the Training Subaward
Budget Form, following the instructions here and in M.330 – PHS 398 Training Budget Form, the prime
grantee must then upload all the Training Subaward Budget Forms to the Training Subaward Budget
Attachment(s) Form.
For all submission methods, the Training Subaward Budget Form with a "Budget Type" of
Subaward/Consortium is used to collect subaward budget data. However, ASSIST and other system-to-
system solutions may present a different interface than the Training Subaward Budget Attachment
Form shown here.
This form accommodates a set number of separate subaward budgets. If you need to add more
subaward budgets than the form allows, include the remaining budgets as part of the "Section F.
Budget Justification" of the project budget.
Regardless of how many subaward/consortium budgets you include, the sum of ALL
subaward/consortium budgets (those attached within the PHS 398 Training Subaward Budget
Attachment(s) Form and those provided as part of the parent budget’s Budget Justification), must be
included in the M.330 - PHS 398 Training Budget, Part B. Consortium Training Costs.
Format:
All attachments, including all Training Subaward Budget Forms and all Budget Justifications, must be
PDF files. The Training Budget Forms are already PDFs when extracted. Do not alter the format.
Hyperlinks and URLs are not allowed unless specified in the funding opportunity announcement.
Content:
On this PHS 398 Training Subaward Budget Attachment(s) Form, you will attach the Training Subaward
Budget files for your application. Each subaward/consortium will complete the Subaward Budget in
accordance with the M.330 - PHS 398 Training Budget Form instructions.
Submitting Subaward Budgets that are not Active for all Periods of the Prime Grant:
The Training Budget Forms do not allow for “empty” budget periods.
Subaward/consortium organizations should complete all budget periods in the Training Subaward
Budget Form for their subaward budgets, aligning the budget period numbers, start dates, and end
dates with the budget periods of the prime grant.
Example: The prime fills out a PHS 398 Training Budget Form with the following periods:
M - 91

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.340 - PHS 398 Training Subaward Budget Attachment(s) Form
l
period 1 - Jan 1, 2017 – Dec 31, 2017
l
period 2 - Jan 1, 2018 – Dec 31, 2018
l
period 3 - Jan 1, 2019 – Dec 31, 2019
l
period 4 - Jan 1, 2020 – Dec 31, 2020
l
period 5 - Jan 1, 2021 – Dec 31, 2021
The budget period numbers and dates should be the same in all Training Subaward Budgets included in
the PHS 398 Training Subaward Budget Attachment(s) Form.
The PHS 398 Training Subaward Budget Forms include several required fields which must be completed
(even for inactive periods) in order to successfully submit the application. Provide the following
information for inactive budget periods in subaward/consortium budgets:
l
Unique Entity Identifier (UEI)
l
Budget Type = Subaward/Consortium
l
Budget Period Start/End Dates (align with budget periods and dates of the prime budget)
l
Explanation of the inactive budget periods in the Budget Justification (of the
subaward/consortium's Training Subaward Budget)
M - 92

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.350 - PHS Additional Indirect Costs Form
M.350 - PHS Additional Indirect Costs
Form
The PHS Additional Indirect Costs Form is used only for
multi-project applications. The applicant organization
responsible for the Overall Component should use this
form to detail its first $25,000 F&A costs on each
subaward organization that leads a component.
View larger image
Quick Links
Introductory Fields
Indirect Costs
Budget Justification
PHS Additional Indirect Cost - Cumulative Budget
Who should use the PHSAdditional Indirect Costs Form:
The PHS Additional Indirect Costs Form is used only for multi-project applications.
The applicant organization responsible for the Overall Component should use this form to detail its first
$25,000 indirect (Facilities and Administrative [F&A]) costs on each subaward organization that leads a
component.
Introductory Fields
Unique Entity Identifier (UEI):
This field is required. Enter the UEI of the applicant organization.
Enter name of Organization:
This field may be pre-populated from the SF 424 (R&R) Form. Enter the name of the organization.
Budget Type:
This field is required. "Project" should be selected.
Budget Period:
This field is required.
Identify the specific budget period (for example, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10).
Start Date:
This field is required and may be pre-populated from the SF 424 (R&R) Form. Enter the
requested/proposed start date of the budget period.
M - 93

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.350 - PHS Additional Indirect Costs Form
End Date:
This field is required. Enter the requested/proposed end date of the budget period.
Indirect Costs
Indirect Cost Type:
Enter the type of indirect cost (e.g., Salary & Wages, Modified Total Direct Costs, etc.) and whether
the cost is off-site. If more than one rate or base is involved for a given type of indirect cost, then
list them as separate entries. If you do not have a current indirect (F&A) rate(s) approved by a
federal agency, indicate “None—will negotiate” and include information for a proposed rate. Use
the Budget Justification in this form if additional space is needed.
Indirect Cost Rate (%):
Enter the most recent indirect cost rate(s) established with the cognizant federal office, or in the
case of for-profit organizations, the rate(s) established with the appropriate agency. If you have a
cognizant/oversight agency and are selected for an award, you must submit your indirect rate
proposal to the NIH awarding IC or to the PHS awarding office for approval. If you do not have a
cognizant/oversight agency, contact the awarding agency.
This field should be entered using a rate such as “55.5.”
Indirect Cost Base ($):
Enter the amount of the base for each indirect cost type.
Funds Requested ($):
Enter the funds requested for each indirect cost type.
See the NIH Grants Policy Statement, Section 7.4: Reimbursement of Facilities and Administrative
Costs for more information.
Total Indirect Costs:
This total will be automatically calculated from the “Funds Requested” column.
Budget Justification
The “Budget Justification” attachment is required.
Attach only one file. Attach this information as a PDF. Hyperlinks and URLs are not allowed unless
specified in the funding opportunity announcement.
Use the Budget Justification to provide the additional information requested in each budget
category identified above and any other information that supports the budget request. The
following budget categories must be justified, where applicable: equipment, travel,
participant/trainee support, and other direct cost categories.
PHS Additional Indirect Cost – Cumulative Budget
Indirect Costs Totals ($):
All values on this form are automatically calculated and the fields pre-populated. They present the
summations of the amounts you entered in the “Indirect Costs” section above, for each of the
M - 94

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.350 - PHS Additional Indirect Costs Form
individual budget periods. Therefore, no data entry is allowed or required to complete this
“Cumulative Budget” section.
If any of the amounts displayed on this form appear to be incorrect, you may correct it by
adjusting one or more of the values that contribute to that total. To make any such corrections,
you will need to revisit the appropriate budget period form(s).
M - 95

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
M.400 - PHS 398 Research Plan Form
The PHS 398 Research Plan form is used only for
research, multi-project, and SBIR/STTR applications.
This form includes fields to upload several attachments,
including the Specific Aims and Research Strategy.
The Research Plan, together with the rest of your
application, should include sufficient information needed
for evaluation of the project, independent of any other
documents (e.g., previous application). Be specific and
informative, and avoid redundancies.
View larger image
Quick Links
Introduction
1. Introduction to Application (for Resubmission and
Revision applications)
Research Plan Section
2. Specific Aims
3. Research Strategy
4. Progress Report Publication List
Other Research Plan Section
5. Vertebrate Animals
6. Select Agent Research
7. Multiple PD/PI Leadership Plan
8. Consortium/Contractual Arrangements
9. Letters of Support
10. Resource Sharing Plan(s)
11. Other Plan(s)
12. Authentication of Key Biological and/or Chemical Resources
Appendix
13. Appendix
Your application should represent a sound approach to the investigation of an important biomedical
research, behavioral research, technological, engineering, or scientific question, and be worthy of
support under the stated criteria of the FOA. It should be self-contained and written with the care and
thoroughness accorded to papers for publication.
M - 96

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Review the application carefully to ensure you have included information essential for evaluation. The
scientific and technical merit of the proposed research is the primary concern for all research supported
by the National Institutes of Health (NIH) and other PHS agencies.
Read all the instructions in the FOA before completing this form to ensure that your application meets
all IC-specific criteria.
Who should use the PHS398 Research Plan Form:
Use the PHS 398 Research Plan Form only if you are submitting a research, multi-project, or SBIR/STTR
application.
Applicants must follow all policies and requirements related to formatting, page limits, and
proprietary information. See the following pages for more information:
l
Format Attachments
l
Page Limits
l
NIH Grants Policy Statement, Section 2.3.11.2: Confidentiality of Information
l
NIH Grants Policy Statement, Section 2.3.11.2.2: The Freedom of Information Act
Introduction
1. Introduction to Application (for Resubmission and Revision applications)
Who must complete the “Introduction to Application” attachment:
An "Introduction to Application" attachment is required only if the type of application is
resubmission or revision or if the FOA specifies that one is needed. An introduction is not allowed
for new or renewal applications.
Descriptions of different types of applications are listed here: NIH Types of Applications.
Format:
Follow the page limits for the introduction in the NIH Table of Page Limits unless otherwise
specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page. Hyperlinks and URLs
may not be used in this section unless specified as allowed in the funding opportunity
announcement.
Content:
Resubmission applications: See specific instructions on the content of the introduction on the
NIH's Resubmission Applications page.
Note: For resubmission applications changing from a single PD/PI to multiple PD/PIs,
changing the number or makeup of the multiple PD/PIs, the applicant must provide a
rationale for the change in the introduction and include the required Multiple PD/PI
Leadership Plan. A rationale for a change from a multiple PD/PI to a single PD/PI application
must also be provided in the introduction.
M - 97
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Competing Revisions: See specific instructions on the content of the introduction on the NIH's
Competing Revisions page.
Additional Instructions for Multi-project:
Overall Component: The “Introduction” attachment is required for all resubmission
and revision applications.
Other Components: The "Introduction” attachment is optional for resubmissions
and revisions applications. Although the “Introduction” attachment is optional, you
may get a system warning if there is no attachment.
Research Plan Section
2. Specific Aims
Who must complete the "Specific Aims" attachment:
The “Specific Aims” attachment is required unless otherwise specified in the FOA.
Format:
Follow the page limits for the Specific Aims in the NIH Table of Page Limits unless otherwise
specified in the FOA. A “Specific Aims” attachment that exceeds the page limit will be flagged as an
error by the Agency upon submission.
Attach this information as a PDF file. See NIH's Format Attachments page. Hyperlinks and URLs
may not be used in this section unless specified as allowed in the funding opportunity
announcement.
Content:
State concisely the goals of the proposed research and summarize the expected outcome(s),
including the impact that the results of the proposed research will have on the research field(s)
involved.
List succinctly the specific objectives of the research proposed (e.g., to test a stated hypothesis,
create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice,
address a critical barrier to progress in the field, or develop new technology).
Additional Instructions for Multi-project:
Overall Component: The "Specific Aims" attachment is required.
Other Components: The "Specific Aims" attachment is required.
3. Research Strategy
Who must complete the "Research Strategy" attachment:
The “Research Strategy” attachment is required.
M - 98

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Format:
Follow the page limits for the Research Strategy in the NIH Table of Page Limits, unless otherwise
specified in the FOA. Although multiple sections of information are required in the Research
Strategy as detailed below, the page limit applies to the entirety of the single "Research Strategy"
attachment.
Attach this information as a PDF file. See NIH's Format Attachments page. Hyperlinks and URLs
may not be used in this section unless specified as allowed in the funding opportunity
announcement.
Content:
Organize the Research Strategy in the specified order and use the instructions provided below
unless otherwise specified in the FOA. Start each section with the appropriate heading –
Significance, Innovation, Approach.
Cite published experimental details in the Research Strategy attachment and provide the full
reference in M.220 - R&R Other Project Information Form, Bibliography and Reference Cited.
Note for Applications Proposing the Use of Human Fetal Tissue: If the use of human fetal
tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy Statement) is
included in the proposed application you must include specific information in the Approach
section of the Research Strategy attachment. See specific instructions below in Section 3.
Approach. This information must be provided regardless of whether Human Subjects research is
proposed or not.
Applications proposing HFTthat do not address these requirements will be administratively
withdrawn. For further information on HFTpolicy refer to the NIHGrants Policy Statement, Section
2.3.7.11 Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research
and Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
Note for Applications Proposing the Involvement of Human Subjects and/or Clinical Trials:
l
Do not duplicate information in the Research Strategy and the PHS Human Subjects and
Clinical Trials Information form. Use the Research Strategy attachment to discuss the overall
strategy, methodology, and analyses of your proposed research. Use the PHS Human Subjects
and Clinical Trials Information form to provide detailed information for human subjects studies
and clinical trials.
l
The PHS Human Subjects and Clinical Trials Information form will capture detailed study
information, including eligibility criteria; inclusion of women, minorities, and individuals across
the lifespan; protection and monitoring plans; and statistical design and power.
l
You are encouraged to refer to information in the PHS Human Subjects and Clinical Trials
Information form as appropriate in your discussion of the Research Strategy (e.g., see Question
2.4 Inclusion of Women and Minorities).
Note for Applicants with Multiple Specific Aims: You may address the Significance, Innovation,
and Approach either for each Specific Aim individually or for all of the Specific Aims collectively.
1. Significance
l
Explain the importance of the problem or critical barrier to progress that the proposed
project addresses.
l
Describe the strengths and weaknesses in the rigor of the prior research (both published
and unpublished) that serves as the key support for the proposed project.
M - 99

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
l
Explain how the proposed project will improve scientific knowledge, technical capability,
and/or clinical practice in one or more broad fields.
Additional Instructions for Multi-project:
Overall and Other Components: Describe how the concepts, methods,
technologies, treatments, services, or preventative interventions that drive this field
will be changed if the proposed aims are achieved.
2. Innovation
l
Explain how the application challenges and seeks to shift current research or clinical
practice paradigms.
l
Describe any novel theoretical concepts, approaches or methodologies, instrumentation
or interventions to be developed or used, and any advantage over existing
methodologies, instrumentation, or interventions.
l
Explain any refinements, improvements, or new applications of theoretical concepts,
approaches or methodologies, instrumentation, or interventions.
3. Approach
l
Describe the overall strategy, methodology, and analyses to be used to accomplish the
specific aims of the project. Describe plans to address weaknesses in the rigor of the prior
research that serves as the key support for the proposed project. Describe the
experimental design and methods proposed and how they will achieve robust and
unbiased results. Include how the data will be collected, analyzed, and interpreted, and
reference any Resource Sharing Plans and the Data Management and Sharing (DMS) Plan,
as appropriate. Resources and tools for rigorous experimental design can be found at the
Enhancing Reproducibility through Rigor and Transparency website.
l
For trials that randomize groups or deliver interventions to groups, describe how your
methods for analysis and sample size are appropriate for your plans for participant
assignment and intervention delivery. These methods can include a group- or cluster-
randomized trial or an individually randomized group-treatment trial. Additional
information is available at the Research Methods Resources webpage.
l
Discuss potential problems, alternative strategies, and benchmarks for success anticipated
to achieve the aims.
l
If the project is in the early stages of development, describe any strategy to establish
feasibility, and address the management of any high risk aspects of the proposed work.
l
Explain how relevant biological variables, such as sex, are factored into research designs
and analyses for studies in vertebrate animals and humans. For example, strong
justification from the scientific literature, preliminary data, or other relevant
considerations, must be provided for applications proposing to study only one sex. Refer
to the NIH Guide Notice on Sex as a Biological Variable in NIH-funded Research for
additional information.
l
Point out any procedures, situations, or materials that may be hazardous to personnel and
the precautions to be exercised. A full discussion on the use of select agents should
appear in the Select Agent Research attachment below.
M - 100

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
l
If research on Human Embryonic Stem Cells (hESCs) is proposed but an approved cell line
from the NIH hESC Registry cannot be chosen, provide a strong justification for why an
appropriate cell line cannot be chosen from the registry at this time.
Special Instructions for Applications Proposing the Use of Human Fetal Tissue: If the use of
human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH Grants Policy
Statement) is included in the proposed application
l
Use the specific heading: “Human Fetal Tissue Research Approach”.
l
Describe the proposed characteristics, procurement, and procedures for the research use of
HFT. The description should be sufficiently detailed to permit meaningful evaluation by NIH.
l
Justify the use of HFT in the proposed research by indicating the following:
l
Why the research goals cannot be accomplished by using an alternative to HFT.
l
What methods were used (e.g. literature review, preliminary data) to determine that
alternatives could not be used.
l
Results from a literature review used to provide justifications.
l
Plans for the treatment of HFT and the disposal of HFT when research is complete.
l
Description of planned written, voluntary, informed consent process for cell/tissue
donation, or description and documentation of process if cells/tissue were already
obtained.
Applications proposing HFT that do not address these requirements will be administratively
withdrawn. For further information on HFTpolicy refer to the NIHGrants Policy Statement, Section
2.3.7.11 Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research
and Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
As applicable, also include the following information as part of the Research Strategy,
keeping within the three sections (Significance, Innovation, and Approach) listed above.
Preliminary Studies for New Applications:
For new applications, include information on preliminary studies. Discuss the PD/PI’s preliminary
studies, data, and or experience pertinent to this application. Except for
Exploratory/Developmental Grants (R21/R33), Small Research Grants (R03), and Academic
Research Enhancement Award (AREA) Grants (R15), preliminary data can be an essential part of a
research grant application and can help to establish the likelihood of success of the proposed
project. Early stage investigators should include preliminary data.
Progress Report for Renewal and Revision Applications:
Note that the Progress Report falls within the Research Strategy and is therefore included in the
page limits for the Research Strategy.
For renewal/revision applications, provide a Progress Report. Provide the beginning and ending
dates for the period covered since the last competitive review. In the Progress Report, you should:
l
Summarize the specific aims of the previous project period and the importance of the
findings, and emphasize the progress made toward their achievement.
l
Explain any significant changes to the specific aims and any new directions, including
changes resulting from significant budget reductions.
l
Discuss previous participant enrollment (e.g., recruitment, retention, inclusion of women,
minorities, children, etc.) for any studies meeting the NIH definition for clinical research.
M - 101

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Use the Progress Report section to discuss, but not duplicate information collected
elsewhere in the application.
Do not include a list of publications, patents, or other printed materials in the Progress Report.
That information will be included in the "Progress Report Publication List" attachment.
Renewal Applications: For renewal applications changing from a single PD/PI to multiple
PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant must provide a
rationale for the change and include the required Multiple PD/PI Leadership Plan. A rationale for a
change from a multiple PD/PI to a single PD/PI application must also be provided.
4. Progress Report Publication List
Who must complete the “Progress Report Publication List” attachment:
A “Progress Report Publication List” attachment is required only if the type of application is
renewal.
Descriptions of different types of applications are listed here: NIH's Types of Applications.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page. Use of hyperlinks and
URLs in this section is not allowed unless specified in these instructions or in the funding
opportunity announcement.
Content:
List the titles and complete references to all appropriate publications, manuscripts accepted for
publication, patents, and other printed materials that have resulted from the project since it was
last reviewed competitively.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions on citing interim research products
and claiming them as products of your NIH award.
Provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the PubMed
Central (PMC) reference number (e.g., PMCID234567) for each of the following:
l
Articles that fall under the Public Access Policy,
l
Articles that were authored or co-authored by the applicant and arose from NIH support,
l
Articles that were authored or co-authored by the applicant and arose from AHRQ
funding provided after February 19, 2016 (see the Guide Notice on Policy for Public
Access to AHRQ-Funded Scientific Publications).
If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of
their authors, indicate “PMC Journal – In Process.” NIHmaintains a list of such journals.
Citations that are not covered by the Public Access Policy, but are publicly available in a free,
online format may include URLs or PubMed ID (PMID) numbers along with the full reference.
Active hyperlinks are not allowed.
Additional Instructions for Multi-project:
Overall and Other Components: If you include a "Progress Report Publication List"
attachment, you can include it in either the Overall Component or within each Other
M - 102

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Component, but do not attach the same information in multiple locations.
Other Research Plan Section
5. Vertebrate Animals
Who must complete the “Vertebrate Animals” attachment:
Include a “Vertebrate Animals” attachment if you answered “Yes” to the question “Are Vertebrate
Animals Used?” on the M.220 - R&R Other Project Information Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Do not use this attachment to circumvent the page limits of the Research Strategy.
Content:
If live vertebrate animals are involved in the project, address each of the following criteria:
1. Description of Procedures: Provide a concise description of the proposed procedures to
be used that involve live vertebrate animals in the work outlined in the “Research
Strategy” attachment. The description must include sufficient detail to allow evaluation of
the procedures. Identify the species, strains, ages, sex, and total numbers of animals by
species, to be used in the proposed work. If dogs or cats are proposed, provide the source
of the animals.
2. Justifications: Provide justification that the species are appropriate for the proposed
research. Explain why the research goals cannot be accomplished using an alternative
model (e.g. computational, human, invertebrate, in vitro).
3. Minimization of Pain and Distress: Describe the interventions including analgesia,
anesthesia, sedation, palliative care and humane endpoints that will be used to minimize
discomfort, distress, pain, and injury.
Each of the criteria must be addressed. Failure to adequately address the criteria may negatively
affect the application’s impact score. In addition to the 3 criteria above, you should also:
l
Identify all project performance (or collaborating) sites and describe the proposed
research activities with vertebrate animals that will be conducted at those sites.
l
Explain when and how animals are expected to be used if plans for the use of animals
have not been finalized.
See the following pages for more information:
l
NIH’s Office of Laboratory Animal Welfare website
l
NIH's Vertebrate Animals Section Worksheet
l
See the NIH Grants Policy Statement, Section 4.1.1: Animal Welfare Requirements (an
applicable Animal Welfare Assurance will be required if the grantee institution does not have
one)
M - 103

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Additional Instructions for Multi-project:
Overall Component: The “Vertebrate Animals” attachment is optional unless
specifically requested in the FOA.
Other Components: Complete the “Vertebrate Animals” section if you answered
“Yes” to the question “Are Vertebrate Animals Used?” on the M.220 - R&R Other
Project Information Form.
6. Select Agent Research
Who must complete the “Select Agent Research” attachment:
Include a “Select Agent Research” attachment if your proposed activities involve the use of select
agents at any time during the proposed project period, either at the applicant organization or at
any performance site.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
For more information:
Select agents are hazardous biological agents and toxins that have been identified by HHS or
theU.S. Department of Agriculture (USDA) as having the potential to pose a severe threat to public
health and safety, to animal and plant health, or to animal and plant products. The Centers for
Disease Control and Prevention (CDC) and the Animal and Plant Health Inspection Service (APHIS)
Select Agent Programs jointly maintain a list of these agents. See the Federal Select Agent
Program website.
See also the NIH Grants Policy Statement, Section 4.1.24.1.1: Select Agents.
Content:
Excluded select agents: If the activities proposed in the application involve only the use of a
strain(s) of select agents which has been excluded from the list of select agents and toxins as per
42 CFR 73.3, the select agent requirements do not apply. Use this “Select Agent Research”
attachment to identify the strain(s) of the select agent that will be used and note that it has been
excluded from this list. The CDC maintains a list of exclusions, which is available on the Select
Agents and Toxins Exclusions website.
Applying for a select agent to be excluded: If the strain(s) is not currently excluded from the list
of select agents and toxins but you have applied or intend to apply to HHS for an exclusion from
the list, use this section to indicate the status of your request or your intent to apply for an
exclusion and provide a brief justification for the exclusion.
All applicants proposing to use select agents: Address the following three points for each site
at which select agent research will take place. Although no specific page limitation applies to this
section, be succinct.
1. Identify the select agent(s) to be used in the proposed research.
2. Provide the registration status of all entities* where select agent(s) will be used.
l
If the performance site(s) is a foreign institution, provide the name(s) of the country
or countries where select agent research will be performed.
M - 104

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
l
*An “entity” is defined in 42 CFR 73.1 as “any government agency (Federal, State, or
local), academic institution, corporation, company, partnership, society, association,
firm, sole proprietorship, or other legal entity.”
3. Provide a description of all facilities where the select agent(s) will be used.
l
Describe the procedures that will be used to monitor possession, use, and transfer of
select agent(s).
l
Describe plans for appropriate biosafety, biocontainment, and security of the select
agent(s).
l
Describe the biocontainment resources available at all performance sites.
7. Multiple PD/PI Leadership Plan
Who must complete the “Multiple PD/PI Leadership Plan” attachment:
Any applicant who designates multiple PD/PIs (on the M.240 - R&R Senior/Key Person Profile
(Expanded) Form) must include a Multiple PD/PI Leadership Plan. For applications designating
multiple PD/PIs, all such individuals must be assigned the PD/PI role on the M.240 - R&R
Senior/Key Profile (Expanded) Form, even those at organizations other than the applicant
organization.
Do not submit a Multiple PD/PI Leadership Plan if you are not submitting a multiple PD/PI
application.
Additional Instructions for Multi-project:
Overall Component: The “Multiple PD/PI Leadership Plan” attachment is required if
more than one PD/PI is specified on the Overall Component's M.240 - R&R
Senior/Key Profile (Expanded) Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
A rationale for choosing a multiple PD/PI approach should be described. The governance and
organizational structure of the leadership team and the research project should be described,
including communication plans, processes for making decisions on scientific direction, and
procedures for resolving conflicts. The roles and administrative, technical, and scientific
responsibilities for the project or program should be delineated for the PD/PIs and other
collaborators.
If budget allocation is planned, the distribution of resources to specific components of the project
or the individual PD/PIs should be delineated in the Multiple PD/PI Leadership Plan. In the event of
an award, the requested allocations may be reflected in a footnote on the Notice of Grant Award.
Resubmission Applications: For resubmission applications changing from a single PD/PI to
multiple PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant must
provide a rationale for the change in the introduction and include the required Multiple PD/PI
Leadership Plan.
Renewal Applications: For renewal applications changing from a single PD/PI to multiple
PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant must provide a
M - 105

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
rationale for the change in the progress report within the research strategy and include the
required Multiple PD/PI Leadership Plan.
For more information:
For background information on the multiple PD/PI initiative, see NIH's Multiple Principal
Investigators page.
8. Consortium/Contractual Arrangements
Who must complete the “Consortium/Contractual Arrangements” attachment:
Include a “Consortium/Contractual Arrangements” attachment if you have consortiums/contracts
in your budget.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Explain the programmatic, fiscal, and administrative arrangements to be made between the
applicant organization and the consortium organization(s). If consortium/contractual activities
represent a significant portion of the overall project, explain why the applicant organization, rather
than the ultimate performer of the activities, should be the grantee.
Note: The signature of the authorized organization representative in M.200 - SF 424 (R&R),
Authorized Representative signifies that the applicant and all proposed consortium participants
understand and agree to the following statement:
The appropriate programmatic and administrative personnel of each organization involved in
this grant application are aware of the agency’s consortium agreement policy and are prepared
to establish the necessary inter-organizational agreement(s) consistent with that policy.
For more information:
Refer to the NIH Grants Policy Statement, Section 15: Consortium Agreements for more
information.
Additional Instructions for Multi-project:
Overall and Other Components: Unless otherwise specified in the FOA, you have
the option to:
l
include a single consolidated “Consortium/Contractual Arrangements”
attachment in the Overall Component, or
l
include component-specific “Consortium/Contractual Arrangements” attachment
(s) within the components that include subawards, or
l
include a “Consortium/Contractual Arrangements” attachment in the Overall
Component and include component-specific attachments within the components
that include subawards. Each filename must be unique.
M - 106

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
9. Letters of Support
Format:
Combine all letters of support into a single PDF file and attach this information here. Do not place
these letters in the Appendix.
Follow the attachment guidelines on NIH's Format Attachments page. Use of hyperlinks and URLs
in Letters of Support is not allowed unless specified in the funding opportunity announcement.
Content:
Attach a file with all letters of support, including any letters necessary to demonstrate the support
of consortium participants and collaborators such as Senior/Key Personnel and Other Significant
Contributors included in the grant application.
Letters should stipulate expectations for co-authorship, and whether cell lines, samples, or other
resources promised in the letter are freely available to other investigators in the scientific
community or will be provided to the particular investigators only.
For consultants, letters should include rate/charge for consulting services and level of effort /
number of hours per budget period anticipated. In addition, letters ensuring access to core
facilities and resources should stipulate whether access will be provided as a fee-for-service.
Material Transfer Agreements may be included in this section.
Letters must focus on the topics listed above and not contain data / figures / tables / graphs,
preliminary data, methods, background and significance details that are expected to be found in
Research Strategy section of the application. Letters of Support serve to describe terms of a
collaboration or consultation and also are not de facto letters of reference from persons not
actively participating in the project. Applications with letters containing such excess information
may be withdrawn from the review process.
Letters are not required for personnel (such as research assistants) not contributing in a
substantive, measurable way to the scientific development or execution of the project.
Do not include consultant biographical sketches in the “Letters of Support” attachment, as
consultant biosketches should be in the “Biographical Sketch” section.
Additional Instructions for Multi-project:
Overall and Other Components: Unless specific instructions are provided in the
FOA, applicants have the option of including the “Letters of Support” attachment in
the Overall Component, Other Components, or both. To avoid duplication, each
letter should appear only once in the application. Letters that apply to the entire
application (or to multiple components) should be presented in the Overall
Component as a single PDF, while letters that apply only to a particular individual
component should be presented in that component as a single PDF.
10. Resource Sharing Plan(s)
Note: Effective for due dates on or after January 25, 2023, Data Management and
Sharing (DMS) Plans are now included in Section 11. Other Plan(s). Plans for Genomic Data
Sharing should be provided as part of the Data Management and Sharing Plan.
M - 107

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Sharing Model Organisms: Regardless of the amount requested, all applications where the
development of model organisms is anticipated are expected to include a description of a specific
plan for sharing and distributing unique model organisms or state why such sharing is restricted or
not possible. For more information, see the NIH Grants Policy Statement, Section 8.2.3.2: Sharing
Model Organisms.
Research Tools:
NIH considers the sharing of unique research resources developed through NIH-sponsored
research an important means to enhance the value and further the advancement of the research.
When resources have been developed with NIH funds, and the associated research findings
published or provided to NIH, it is important that they be made readily available for research
purposes to qualified individuals within the scientific community. For more information, see the
Research Tools Policy on the NIH Scientific Data Sharing Website and the NIH Grants Policy
Statement, Section 8.2.3: Sharing Research Resources.
11. Other Plan(s)
Who Must Complete This Section: Refer to the list of NIH activity codes subject to the DMS
Policy and your Funding Opportunity Announcement to determine if your application is required
to provide an attachment and address a Data Management and Sharing (DMS) Plan. Applicants
proposing to conduct research that will generate scientific data are subject to the NIH Data
Management and Sharing Policy and must attach a Data Management and Sharing (DMS) Plan.
Scientific data is defined as the recorded factual material commonly accepted in the scientific
community as of sufficient quality to validate and replicate research findings, regardless of
whether the data are used to support scholarly publications. Scientific data includes any data
needed to validate and replicate research findings. Scientific data does not include laboratory
notebooks, preliminary analyses, completed case report forms, drafts of scientific papers, plans for
future research, peer reviews, communications with colleagues, or physical objects such as
laboratory specimens.
The NIH Genomic Data Sharing Policy expects applicants seeking funding for research that
generates large-scale human or non-human genomic data to provide a plan for sharing of these
data as part of their DMS Plan.
Applicants subject to both the NIH Data Management and Sharing Policy and the NIH Genomic
Data Sharing Policy must attach a single Plan including elements for both policies. For more on
applicability of each policy, see research subject to the NIH Data Management and Sharing Policy
and the research subject to the NIH Genomic Data Sharing Policy.
Format: Attach this information as a PDF file. See NIH's Format Attachments page.
A sample format is provided on the Data Management and Sharing Plan Format Page to assist
applicants with preparation of this attachment. Do not include hyperlinks in this attachment.
Recommended not to exceed two pages.
Content: Follow the expectations of the NIH Policy for Data Management and Sharing and
address the Elements of an NIH Data Management and Sharing Plan described below.
M - 108
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Additional expectations: A Data Management and Sharing Plan should reflect the proposed
approach at the time the application is prepared. For some programs and data types, NIH and/or
NIH Institutes, Centers, Offices, or programs have developed additional data sharing requirements
(e.g., specifying which scientific data to share, relevant standards, repository selection, timelines)
that apply and should be reflected in a Plan. These additional requirements may be listed on NIH
Institute and Center Data Sharing Policies or in specific funding opportunity announcements. Note
that some NIH Institutes, Centers, Officers, or programs have developed additional expectations
for sharing genomic data that may be listed on NIH Institute and Center Genomic Data Sharing
Expectations or in specific funding opportunity announcements.
Elements of a Data Management and Sharing Plan:
Data Type: Briefly describe the scientific data to be managed, preserved, and shared,
including a general summary of the types and estimated amount of scientific data to be
generated and a description of which scientific data from the project will be preserved and
shared as well as the rationale for doing so. Briefly list the metadata, other relevant data, and
any associated documentation (e.g., study protocols and data collection instruments) that will
be made accessible to facilitate interpretation of the scientific data.
Related Tools, Software and/or Code: State whether specialized tools are needed to access or
manipulate shared scientific data to support replication or reuse, and name(s) of the needed
tool(s) and software. If specialized tools or software are needed, provide the name(s) of the
needed tool(s) and software and specify how they can be accessed.
Standards: State what common data standards will be applied to the scientific data and
associated metadata to enable interoperability of datasets and resources (e.g., data formats,
data dictionaries, data identifiers, definitions, unique identifiers, and other data
documentation), and provide the name(s) of the data standards that will be applied and
describe how these data standards will be applied to the scientific data generated by the
research proposed in this project. If applicable, indicate that no consensus standards exist.
Data Preservation, Access, and Associated Timelines: Provide plans and timelines for data
preservation and access, including the name of the repository(ies) where scientific data and
metadata arising from the project will be archived (do not include hyperlinks); how the
scientific data will be findable and identifiable, i.e., via a persistent unique identifier or other
standard indexing tools; and when (i.e., no later than time of an associated publication or end
of the performance period, whichever comes first) the scientific data will be made available to
other users (e.g., the larger research community, institutions, and/or the broader public) and
for how long. See Selecting a Data Repository on the NIH Scientific Data Sharing website.
Access, Distribution, or Reuse Considerations: NIH expects that in drafting Plans, researchers
maximize the appropriate sharing of scientific data generated from NIH-funded or conducted
research, consistent with privacy, security, informed consent, and proprietary issues. Describe
and justify any applicable factors affecting subsequent access, distribution, or reuse of
scientific data related to informed consent, privacy and confidentiality protections, any
restrictions imposed by federal, Tribal, or state laws, regulations, or policies, or existing or
anticipated agreements, or any other considerations that may limit the extent of data sharing.
See Frequently Asked Questions for examples of justifiable reasons for limiting sharing of
data. State whether access to the scientific data will be controlled (i.e., made available by a
data repository only after approval).
Genomic Data Sharing Policy: For proposed research subject to the GDS Policy, state
whether data, including genomic summary results, will be made available through controlled
M - 109
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
or unrestricted access; see instructions for describing Genomic Summary Results in Data
Management and Sharing Plans.
If generating scientific data derived from humans, describe how the privacy, rights, and
confidentiality of human research participants will be protected (e.g., through de-
identification, Certificates of Confidentiality, and other protective measures). See NIH’s
Scientific Data Sharing page for additional information on protecting human research
participant privacy when sharing data.
Genomic Data Sharing Policy: For proposed research generating human genomic data
within the scope of the GDS Policy, applicants should complete the Data Management and
Sharing Plan anticipating sharing according to the assurances of the Institutional
Certification.
If there is any element of the Institutional Certification that the institution (in consultation
with the IRB) has determined cannot be met, please state which element and provide a
detailed explanation for why the element cannot be met. In such cases, the data management
and sharing plan should describe how genomic data will be shared to the maximal extent
possible (for example, sharing data in a summary format).
Oversight of Data Management and Sharing: Describe how compliance with the Plan will be
monitored and managed, frequency of oversight, and by whom at the applicant institution
(e.g., titles, roles).
For more information on developing a Data Management and Sharing Plan, see Writing a
Data Management and Sharing Plan on the NIH Scientific Data Sharing website.
For more information on the DMS Policy, including expectations for data management and
sharing, protecting research participant privacy, and identifying data repositories, see the NIH Data
Management and Sharing Policy on the NIH Scientific Data Sharing website and the NIH Grants
Policy Statement, Section 8.2.3.1: Data Sharing Policy. See also Frequently Asked Questions for
additional information on the DMS Policy on these and other topics.
For more information on the GDS Policy see the NIH Genomic Data Sharing Policy on the NIH
Scientific Data Sharing website and the NIH Grants Policy Statement, Section 8.2.3.3: Genomic
Data Sharing (GDS) Policy/ Policy for Genome-Wide Association Studies (GWAS).
Additional Instructions for Multi-project:
Overall Component Include a single consolidated “Data Management and
Sharing Plan” in the Overall Component.
Other Components: Do not include a “Data Management and Sharing Plan”
within other components. Any component-specific information should be described
within the overall “Data Management and Sharing Plan” attachment in the Overall
Component.
12. Authentication of Key Biological and/or Chemical Resources
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
M - 110
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
Content:
If applicable to the proposed science, briefly describe methods to ensure the identity and validity
of key biological and/or chemical resources used in the proposed studies. A maximum of one page
is suggested.
For more Information:
Key biological and/or chemical resources are characterized as follows.
l
Key biological and/or chemical resources may or may not have been generated with NIH
funds and: 1) may differ from laboratory to laboratory or over time; 2) may have qualities
and/or qualifications that could influence the research data; and 3) are integral to the
proposed research. These include, but are not limited to, cell lines, specialty chemicals,
antibodies, and other biologics.
l
Standard laboratory reagents that are not expected to vary do not need to be included in
the plan. Examples are buffers and other common biologicals or chemicals.
l
See NIH's page on Rigor and Reproducibility for more information.
Appendix
13. Appendix
Refer to the FOA to determine whether there are any special appendix instructions for your
application. See the updated NIH Guide Notice on the Appendix Policy.
Additional Instructions for Multi-project:
Overall and Other Components: The "Appendix” attachment is optional.
Format:
A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 allowable appendix
attachments are needed, combine the remaining information into attachment #10.
Use filenames for attachments that are descriptive of the content.
A summary sheet listing all of the items included in the Appendix is encouraged but not required.
When including a summary sheet, it should be included in the first appendix attachment.
Content:
The only allowable appendix materials are:
l
Blank data collection forms, blank survey forms, and blank questionnaire forms - or
screenshots thereof
l
Simple lists of interview questions
Note: In your blank forms and lists, do not include items such as: data, data
compilations, lists of variables or acronyms, data analyses, publications, manuals,
instructions, descriptions or drawings/figures/diagrams of data collection methods or
machines/devices.
M - 111

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.400 - PHS 398 Research Plan Form
l
Blank informed consent/assent forms
l
Other items only if they are specified in the FOA as allowable appendix materials
No other items are allowed in the Appendix. Simply relocating disallowed materials to other parts
of the application will result in a noncompliant application.
Some FOAs may have different instructions for the Appendix. Always follow the instructions in
your FOA if they conflict with these instructions.
Note: Applications will be withdrawn and not reviewed if they do not follow the appendix
requirements in these instructions or in your FOA.
Information that expands upon or complements information provided in any section of the
application – even if it is not required for the review – is not allowed in the Appendix unless it is
listed in the allowed appendix materials above or in your FOA. For example, do not include
material transfer agreements (MTA) in the appendix unless otherwise specified in the FOA.
For more information:
l
The NIH Guide Notice on Reminder: NIH Applications Must Be Complete and Compliant
With NIH Policy and Application Instructions At Time of Submission.
l
Failure of reviewers to address non-required appendix materials in their reviews is not an
acceptable basis for an appeal of initial peer review. For more information, see the NIH
Grants Policy Statement, Section 2.4.2: Appeals of Initial Scientific Review.
l
Appendix Policy Frequently Asked Questions
M - 112

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
M.410 - PHS 398 Career Development
Award Supplemental Form
The PHS 398 Career Development Award Supplemental
Form is used only for career development applications
and multi-project applications with an "Indiv. Career
Dev" Component.
This form includes fields to upload several attachments
including the Specific Aims, Research Strategy, and
Candidate Background and Goals.
See NIH's Reference Letters page for information
including instructions for referees and how to submit
letters.
The attachments in this form, together with the rest of
your application, should include sufficient information
needed for evaluation of the project and the candidate,
independent of any other documents (e.g., previous
application). Be specific and informative, and avoid
redundancies.
View larger image
Quick Links
Introduction
1. Introduction to Application (for Resubmission and Revision applications)
Candidate Section
2. Candidate Information and Goals for Career Development
Research Plan Section
3. Specific Aims
4. Research Strategy
5. Progress Report Publication List (for Renewal applications)
6. Training in the Responsible Conduct of Research
Other Candidate Information Section
7. Candidate's Plan to Provide Mentoring
Mentor, Co-Mentor, Consultant, Collaborators Section
8. Plans and Statements of Mentor and Co-Mentor(s)
9. Letters of Support from Collaborators, Contributors, and Consultants
Environment and Institutional Commitment to Candidate Section
M - 113

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
10. Description of Institutional Environment
11. Institutional Commitment to Candidate's Research Career Development
12. Description of Candidate’s Contribution to Program Goals
Other Research Plan Sections
13. Vertebrate Animals
14. Select Agent Research
15. Consortium/Contractual Arrangements
16. Resource Sharing
17. Other Plan(s)
18. Authentication of Key Biological and/or Chemical Resources
Appendix
19. Appendix
Citizenship
20. U.S. Citizen or Non-Citizen National?
Who should use the PHS 398 Career Development Award Supplemental Form:
Use the PHS 398 Career Development Award Supplemental Form only if you are submitting a career
development application or a multi-project application that has an "Indiv. Career Dev" Component.
Some sections of the PHS 398 Career Development Award Supplemental Form are required for all
career development award applications, while others are to be used only when required by the FOA.
Read all the instructions in the FOA before completing this section to ensure your application meets all
IC-specific criteria.
Applicants must follow all policies and requirements related to formatting, page limits, and
proprietary information. See the following pages for more information:
l
Format Attachments
l
Page Limits
l
NIH Grants Policy Statement, Section 2.3.11.2: Confidentiality of Information
l
NIH Grants Policy Statement, Section 2.3.11.2.2: The Freedom of Information Act
M - 114
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Introduction
1. Introduction to Application (for Resubmission and Revision applications)
Who must complete the “Introduction to Application” attachment:
An "Introduction to Application" attachment is required only if the type of application is
resubmission or revision. An introduction is not allowed for new or renewal applications.
Descriptions of different types of applications are listed here: NIH Types of Applications.
Format:
Follow the page limits for the Introduction in the NIH Table of Page Limits unless otherwise
specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Resubmission applications: See specific instructions on the content of the Introduction on the
NIH's Resubmission Applicationspage.
Competing Revisions: See specific instructions on the content of the Introduction on the NIH's
Competing Revisionspage.
Additional Instructions for Multi-project:
Other Components: The "Introduction” attachment is optional for resubmissions
and revisions applications. Although the “Introduction” attachment is optional, you
may get a system warning if there is no attachment.
Candidate Section
2. Candidate Information and Goals for Career Development
Who must complete the "Candidate Information and Goals for Career Development"
attachment:
The “Candidate Information and Goals for Career Development” attachment is required.
Format:
Follow the page limits for Candidate Information and Goals for Career Development in the
NIHTable of Page Limits, unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Organize your attachment into three sections, following the headings and specified order below,
and discuss each of the points listed below. Start each section with the appropriate section
heading – Candidate’s Background, Career Goals and Objectives, and Candidate’s Plan for Career
M - 115

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Development/Training Activities During Award Period. Also include any additional information
requested in the FOA.
Candidate’s Background:
l
Describe your past scientific history, indicating how the award fits into past and future
research career development.
l
If there are consistent themes or issues that have guided previous work, these should be
made clear. Alternatively, if your work has changed direction, indicate the reasons for the
change.
Career Goals and Objectives:
l
Describe your short-term and long-term career development goals.
l
Justify the need for the award by describing how the career development award will
enable you to develop and/or expand your research career.
l
If applicable (e.g., K24), describe how this award will help you to serve as a mentor to early
career investigators.
Candidate’s Plan for Career Development/Training Activities During Award Period:
l
Describe the new or enhanced research skills and knowledge you will acquire as a result of
the proposed award, including, as applicable, expertise in rigorous research design,
experimental methods, quantitative approaches and data analysis and interpretation.
l
For non-mentored career development awards, describe any planned release from
teaching, administrative, and/or clinical duties that will help you focus on your research
activities, and if applicable, your mentoring activities.
l
For mentored career development awards, describe any structured activities that are part
of the developmental plan, such as coursework or workshops that will help you learn new
techniques or develop needed professional skills.
l
Briefly discuss each of the activities, other than research, in which you expect to
participate.
l
For each activity, other than research, explain how it relates to the proposed research and
to the career development plan. Indicate the percentage of time to be dedicated to each
activity by year, expressed in person months. For more information about calculating
person months, see NIH's Frequently Asked Questions on Person Months.
l
You are encouraged to include a timeline, including plans to apply for subsequent grant
support.
Research Plan Section
A Research Plan is required for all types of individual career development awards.
The information in these introductory paragraphs to the Research Plan Section applies to all four
Research Plan attachments: Specific Aims, Research Strategy, Progress Report Publication List, and
Training in the Responsible Conduct of Research.
M - 116

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
The Research Plan is a major part of the overall career development goal. It is important to relate
the proposed research to the candidate's scientific career goals. Describe how the research,
coupled with other developmental activities, will provide the experience, knowledge, and skills
necessary to achieve the objectives of the career development plan. Also describe how the
research and other developmental activities will enable the candidate to launch and conduct an
independent research career or enhance an established research career.
For most types of research, the Research Plan Section should include:
l
a specific hypothesis,
l
a list of the specific aims and objectives that will be used to examine the hypothesis,
l
a description of the methods/approaches/techniques to be used in each aim,
l
a discussion of possible problems and how they will be managed, and
l
alternative approaches that might be tried if the initial approaches do not work.
A Career Development Award (CDA) Research Plan is expected to be tailored to the experience
level of the candidate and to allow him/her to develop the necessary skills needed for further
career advancement. Reviewers will evaluate the plan accordingly. The plan should be achievable
within the requested time period. Pilot or preliminary studies and routine data gathering are
generally not appropriate as the sole part(s) of a CDA Research Plan.
Although candidates for mentored career development awards are expected to write the Research
Plan, the mentor should review a draft of the plan and discuss it in detail with the candidate.
Review by other knowledgeable colleagues is also helpful. Although it is understood that CDA
applications do not require the extensive detail usually incorporated into regular research grant
applications, a fundamentally sound Research Plan that includes a reasonably detailed Research
Strategy section should be provided.
3. Specific Aims
Who must complete the “Specific Aims” attachment:
The "Specific Aims" attachment is required unless otherwise specified in the FOA.
Format:
Follow the page limits for the Specific Aims in the NIHTable of Page Limits, unless otherwise
specified in the FOA. A “Specific Aims” attachment that exceeds the page limit will be flagged as an
error by the Agency upon submission.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
State concisely the goals of the proposed research and summarize the expected outcome(s),
including the impact that the results of the proposed research will have on the research field(s)
involved.
List succinctly the specific objectives of the research proposed (e.g., to test a stated hypothesis,
create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice,
address a critical barrier to progress in the field, or develop new technology).
M - 117

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
4. Research Strategy
Who must complete the "Research Strategy" attachment:
The “Research Strategy” attachment is required.
Format:
Follow the page limits for the Research Strategy in the NIHTable of Page Limits, unless otherwise
specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Organize the Research Strategy in the specified order and use the instructions provided below.
Start each section with the appropriate heading – Significance, Innovation, Approach.
Cite published experimental details in the Research Strategy section and provide the full reference
in M.220 - R&R Other Project Information Form, Bibliography and References Cited.
In general, less detail will be expected in descriptions of research planned for the future years of
the proposed CDA compared to the initial years’ descriptions. However, sufficient detail should be
provided to enable peer reviewers to determine that the plans for those years, including the
approach to be used, are worthwhile and are likely to enable the candidate to achieve the
objectives of the Research Plan.
Note for mentored CDA applications: Explain the relationship between the candidate’s research
on the CDA and the mentor’s ongoing research program.
Note for non-mentored CDA applications: In general, non-mentored CDA applicants are
expected to have independent, peer-reviewed research support. Applications should include a
brief description of currently funded research, along with a more extensive description of any new
research to be supported by the CDA.
Note for Applications Proposing the Use of Human Fetal Tissue:
If the use of human fetal tissue obtained from elective abortions (HFT) (as defined in the NIH
Grants Policy Statement) is included in the proposed application you must include specific
information in the Approach section of the Research Strategy attachment. See specific instructions
below in Section 3. Approach. This information must be provided regardless of whether Human
Subjects research is proposed or not. These specific instructions do not apply to institutional
career development applications (e.g. K12, KL2).
Note for Applications Proposing the Involvement of Human Subjects and/or Clinical Trials:
l
Use the Research Strategy section to discuss the overall strategy, methodology, and analyses of
your proposed research, but do not duplicate information collected in the PHS Human Subjects
and Clinical Trials Information form.
l
The PHS Human Subjects and Clinical Trials Information form will capture detailed study
information, including eligibility criteria; inclusion of women, minorities, and individuals across
the lifespan; protection and monitoring plans; and statistical design and power.
l
You are encouraged to refer to information in the PHS Human Subjects and Clinical Trials
Information form as appropriate in your discussion of the Research Strategy (e.g., see Question
2.4 Inclusion of Women and Minorities).
M - 118

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Note for Applicants with Multiple Specific Aims: You may address the Significance, Innovation,
and Approach either for each Specific Aim individually or for all of the Specific Aims collectively.
1. Significance
l
Explain the importance of the problem or critical barrier to progress that the proposed
project addresses.
l
Describe the strengths and weaknesses in the rigor of the prior research (both published
and unpublished) that serves as the key support for the proposed project.
l
Explain how the proposed project will improve scientific knowledge, technical capability,
and/or clinical practice in one or more broad fields.
l
Describe how the concepts, methods, technologies, treatments, services, or preventative
interventions that drive this field will be changed if the proposed aims are achieved.
2. Innovation
l
Explain how the application challenges current research or clinical practice paradigms.
l
Describe any novel theoretical concepts, approaches or methodologies, instrumentation
or interventions to be developed or used, and any advantage over existing
methodologies, instrumentation, or interventions.
3. Approach
l
Describe the overall strategy, methodology, and analyses to be used to accomplish the
specific aims of the project. Describe plans to address weaknesses in the rigor of the prior
research that serves as the key support for the proposed project. Describe the
experimental design and methods proposed and how they will achieve robust and
unbiased results. Unless addressed separately in the Resource Sharing Plan section,
include how the data will be collected, analyzed, and interpreted, as well as any resource
sharing plans and the Data Management and Sharing (DMS)Plan as appropriate.
Resources and tools for rigorous experimental design can be found at the Enhancing
Reproducibility through Rigor and Transparency website.
l
For trials that randomize groups or deliver interventions to groups, describe how your
methods for analysis and sample size are appropriate for your plans for participant
assignment and intervention delivery. These methods can include a group- or cluster-
randomized trial or an individually randomized group-treatment trial. Additional
information is available at the Research Methods Resources webpage.
l
Discuss potential problems, alternative strategies, and benchmarks for success anticipated
to achieve the aims.
l
If the project is in the early stages of development, describe any strategy to establish
feasibility, and address the management of any high risk aspects of the proposed work.
l
Explain how relevant biological variables, such as sex, are factored into research designs
and analyses for studies in vertebrate animals and humans. For example, strong
justification from the scientific literature, preliminary data, or other relevant
considerations, must be provided for applications proposing to study only one sex. Refer
to NIH Guide Notice on Sex as a Biological Variable in NIH-funded Research for additional
information.
M - 119

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
l
Point out any procedures, situations, or materials that may be hazardous to personnel and
precautions to be exercised. A full discussion on the use of select agents should appear in
the Select Agent Research section below.
l
If research on Human Embryonic Stem Cells (hESCs) is proposed but an approved cell line
from the NIH hESC Registry cannot be chosen, provide a strong justification for why an
appropriate cell line cannot be chosen from the registry at this time.
l
Special Instructions for Applications Proposing the Use of Human Fetal Tissue:
If the use of human fetal tissue obtained from elective abortions (HFT) (as defined in the
NIH Grants Policy Statement) is included in the proposed application:
l
Use the specific heading: “Human Fetal Tissue Research Approach”.
l
Describe the proposed characteristics, procurement, and procedures for the
research use of HFT. The description should be sufficiently detailed to permit
meaningful evaluation by NIH.
l
Justify the use of HFT in the proposed research by indicating the following:
l
Why the research goals cannot be accomplished by using an alternative
to HFT.
l
What methods were used (e.g. literature review, preliminary data) to
determine that alternatives could not be used.
l
Results from a literature review used to provide justifications.
l
Plans for the treatment of HFT and the disposal of HFT when research is
complete.
l
Description of planned written, voluntary, informed consent process for
cell/tissue donation, or description and documentation of process if
cells/tissue were already obtained.
l
Note: These specific instructions do not apply to institutional career
development applications (e.g. K12).
l
Applications proposing HFT that do not address these
requirements will be administratively withdrawn. For further
information on HFTpolicy refer to the NIHGrants Policy Statement,
Section 2.3.7.11 Human Fetal Tissue from Elective Abortions,
Section 4.1.14 Human Fetal Tissue Research and Section 4.1.14.2
Human Fetal Tissue from Elective Abortions.
l
If you are proposing to gain clinical trial research experience (i.e., you will not be leading
an independent clinical trial), briefly describe your role on the clinical trial.
As applicable, also include the following information as part of the Research Strategy,
keeping within the three sections (Significance, Innovation, and Approach) listed above.
Preliminary Studies (for New Applications):
For new applications, include information on preliminary studies. Discuss the PD/PI’s
preliminary studies, data, and or experience pertinent to this application.
M - 120

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Progress Report (for Renewal and Revision Applications):
Most career development applicants will not complete this attachment. However, if you are
required to do so, note that the Progress Report falls within the Research Strategy and is
therefore included in the page limits for the Research Strategy.
For renewal/revision applications, provide a Progress Report. Provide the beginning and
ending dates for the period covered since the last competitive review. In the Progress Report,
you should:
l
Summarize the specific aims of the previous project period and the importance of the
findings, and emphasize the progress made toward their achievement.
l
Explain any significant changes to the specific aims and any new directions, including
changes resulting from significant budget reductions.
l
Discuss previous participant enrollment (e.g., recruitment, retention, inclusion of women,
minorities, children, etc.) for any studies meeting the NIH definition for clinical research.
Use the Progress Report section to discuss, but not duplicate information collected
elsewhere in the application.
Do not include a list of publications, patents, or other printed materials in the Progress
Report. That information should be included in the "Progress Report Publication List"
attachment.
5. Progress Report Publication List (for Renewal applications)
Who must complete the “Progress Report Publication List” attachment:
A “Progress Report Publication List” attachment is required only if the type of application is
renewal. Most career development applicants will not complete this attachment.
Descriptions of different types of applications are listed here: NIH's Types of Applications.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
List the titles and complete references to all appropriate publications, manuscripts accepted for
publication, patents, and other printed materials that have resulted from the project since it was
last reviewed competitively.
You are allowed to cite interim research products. Note: interim research products have specific
citation requirements. See related Frequently Asked Questions on citing interim research products
and claiming them as products of your NIH award.
Provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the PubMed
Central (PMC) reference number (e.g., PMCID234567) for the following:
l
Articles that fall under the Public Access Policy,
l
Articles that were authored or co-authored by the applicant and arose from NIH support,
l
Articles that were authored or co-authored by the applicant and arose from AHRQ
funding provided after February 19, 2016 (see the Guide Notice on Policy for Public
Access to AHRQ-Funded Scientific Publications).
M - 121

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of
their authors, indicate “PMC Journal – In Process.” NIHmaintains a list of such journals.
Citations that are not covered by the Public Access Policy, but are publicly available in a free,
online format may include URLs or PubMed ID (PMID) numbers along with the full reference.
Additional Instructions for Multi-project:
Overall and Other Components: If you include a “Progress Report Publication List”
attachment, you can include it in either the Overall Component or within each Other
Component, but do not attach the same information in multiple locations.
6. Training in the Responsible Conduct of Research
Who must complete the "Training in the Responsible Conduct of Research" attachment:
The “Training in the Responsible Conduct of Research” attachment is required.
Format:
Follow the page limits for the Training in the Responsible Conduct of Research in the NIHTable of
Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Mentored CDA applications should describe a plan to acquire instruction in the responsible
conduct of research (RCR).
Non-mentored (independent) CDAapplications should describe a plan to obtain or provide
instruction in RCR, depending on your level of experience with RCR.
Attach a description of plans for obtaining or providing instruction in RCR. This section should
document prior instruction or participation in RCR training during the applicant’s current career
stage (including the date instruction was last completed). This section should also propose plans
to either receive instruction or provide instruction (e.g., to participate as a course lecturer) to meet
the frequency requirement of RCR training (see the “For more information section” below).
The plan must address the five required instructional components outlined in the NIH Policy on
Instruction in the Responsible Conduct of Research (RCR), as more fully described in the NIH
Grants Policy Statement, Section 12.4.1.4: Training in the Responsible Conduct of Research.
1. Format: Describe the required format of instruction, i.e., face-to-face lectures,
coursework, and/or real-time discussion groups (a plan with only on-line instruction is not
acceptable).
2. Subject Matter: Describe the breadth of subject matter (e.g., conflict of interest,
authorship, data management, human subjects and animal use, laboratory safety,
research misconduct, research ethics).
3. Faculty Participation: Describe the role of the mentor(s) and other faculty involvement in
the instruction.
4. Duration of Instruction: Describe the number of contact hours of instruction, taking into
consideration the duration of the program.
M - 122

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
5. Frequency of Instruction: Instruction must occur during each career stage and at least
once every four years. Document any prior instruction during the applicant’s current
career stage, including the inclusive dates instruction was last completed.
The plan may include career stage-appropriate individualized instruction or independent scholarly
activities. Instruction and activities should enhance the applicant’s understanding of ethical issues
related to their specific research activities and the societal impact of that research. The role of the
mentor in RCR instruction must be described.
Renewal Applications: Describe the RCR instruction activities undertaken during the previous
project period as well as future plans for RCR instruction.
For more information:
See the NIH Grants Policy Statement, Section 12.4.1.4: Training in the Responsible Conduct of
Research.
Other Candidate Information Section
7. Candidate’s Plan to Provide Mentoring
Who must complete the “Candidate’s Plan to Provide Mentoring” attachment:
Include the “Candidate’s Plan to Provide Mentoring” attachment only when required by the FOA,
(e.g., K05 and K24).
Format:
Follow the page limits for the Candidate’s Plan to Provide Mentoring in the NIH Table of Page
Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
The plan should provide information about both the candidate’s commitment to serve as a mentor
to other investigators and the candidate’s previous mentoring activities. State the candidate’s
proposed percent effort commitment to the mentoring plan, expressed in person months. For
more information about calculating person months, see NIH's Frequently Asked Questions on
Person Months.
Describe proposed mentoring activities: Describe the setting for mentoring and provide
information about the available pool of mentees with appropriate backgrounds and similar
interests in science as the candidate. Include information sufficient for reviewers to evaluate the
quality of the proposed mentoring experience, including the professional levels of mentees and
the frequency and kinds of mentoring interactions between the candidate and mentees. Describe
the productivity of the mentoring relationship for the scientific development of the new scientists
as judged by their publications and current research activities.
Describe past mentoring activities: Include sufficient information on the candidate’s past
mentees so that reviewers can evaluate the quality of prior mentoring experiences. Include
information such as the professional levels of mentees, and the frequency and kinds of mentoring
interactions between the candidate and mentees.
M - 123

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Senior level (K05) candidates: Describe any financial and material support from your own
funded research and research resources that will be available to your mentees.
Mentor, Co-Mentor, Consultant, Collaborators Section
8. Plans and Statements of Mentor and Co-Mentor(s)
Who must complete the “Plans and Statements of Mentor and Co-Mentor(s)” attachment:
Any candidate applying for a mentored CDA (see Summary of Career Development Award
Mechanisms table) must include a "Plans and Statement of Mentor and Co-Mentor(s)" attachment.
All mentored career development applications should identify any and all co-mentors involved
with the proposed research and career development program. The mentor and each co-mentor
must provide a statement as described below.
Format:
Follow the page limits for the Plans and Statements of Mentor and Co-mentor(s) in the NIHTable
of Page Limits unless otherwise specified in the FOA.
The plans and statements must be appended together and uploaded as a single PDF file. See NIH's
Format Attachments page.
Content:
The mentor and co-mentor(s) (if applicable) must each document their role and willingness to
participate in the project, and explain how they will contribute to the development of the
candidate's research career. Each statement should include all of the following:
1. The plan for the candidate's training and research career development. Include
information not only about research, but also about other developmental activities, such
as seminars, scientific meetings, training in RCR, and presentations. Discuss expectations
for publications over the entire period of the proposed project. Define what aspects of the
proposed research project the candidate will be allowed to continue to pursue as part of
his/her independent research program.
2. The source of anticipated support for the candidate’s research project for each year of the
award period.
3. The nature and extent of supervision and mentoring of the candidate, and commitment to
the candidate's development that will occur during the award period.
4. The candidate's anticipated teaching load for the award period (number and types of
courses or seminars), clinical responsibilities, committee and administrative assignments,
and the portion of time available for research.
5. A plan for transitioning the candidate from the mentored stage of his/her career to the
independent investigator stage by the end of the project period of the award. Describe
the mentor's (or co-mentor’s) previous experience as a mentor, including type of
mentoring (e.g., graduate students, career development awardees, postdoctoral fellows),
number of persons mentored, and career outcomes.
Note for co-mentor statements: Co-mentors must also address the nature of their role in the
career development plan and how the responsibility for the candidate’s development is shared
M - 124

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
with the mentor. Describe respective areas of expertise and how they will be combined to enhance
the candidate’s development. Also describe the nature of any resources that will be committed to
this CDA.
Note: If the applicant is proposing to gain experience in a clinical trial as part of his or her
research career development, then the mentor or a member of the mentoring team should
include information in the statement to document leadership of the clinical trial (in addition to the
information above). Include the following:
l
Source of funding;
l
ClinicalTrials.gov Identifier (e.g., NCT87654321), if applicable;
l
A description of how your expertise is appropriate to guide the applicant in any proposed
clinical trials research experience; and
l
A statement/attestation that the mentor will be responsible for the clinical trial.
l
The mentor must have primary responsibility for leading and overseeing the trial and
must describe how she/he will provide this oversight (be careful not to overstate the
candidate’s responsibilities).
l
Include details on the specific roles/responsibilities of the applicant and mentor, keeping
in mind that the terms of a CDA award do not always permit the candidate to lead a
clinical trial.
Do not place these statements from the mentor(s) and co-mentor(s) in the Appendix.
9. Letters of Support from Collaborators, Contributors, and Consultants
Note that letters of support are not the same as letters of reference (also known as reference
letters), which are required for some K applications. For more information about letters of
reference, see the NIH's Reference Letters page.
From whom are letters of support required? From whom are letters not required?
Letters of support from collaborators, contributors, and consultants will be required for any such
person who will contribute to the scientific development or execution of CDA application’s
proposed project. Follow the requirements for letters of support as listed in the FOA.
Letters are not required for personnel (such as research assistants) not contributing in a
substantive, measurable way to the scientific development or execution of the project.
Format:
Follow the page limits for the Letters of Support from Collaborators, Contributors, and Consultants
in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach all appropriate letters of support. The letters must be appended together and uploaded as
a single PDF file. See NIH's Format Attachments page. Use of hyperlinks and URLs in this section is
not allowed unless specified in the funding opportunity announcement.
Content:
Letters from consultants should include rates/charges for consulting services.
Mentored CDA applications should identify collaborators, contributors, and consultants involved
with the proposed research and career development program, and not already included in the
“Plans and Statements of Mentor(s) and Co-Mentor(s)” section. Letters should briefly describe their
M - 125

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
anticipated contributions and document their role and willingness to participate in the project. The
letters should also briefly describe research materials, data, guidance, or advice each person will
provide.
Non-mentored CDA applications should include letters from collaborators, consultants, and
contributors. Letters should list proposed roles and document their willingness to participate in
the project. The letters should also briefly describe research materials, data, guidance, or advice
each person will provide.
Environment and Institutional Commitment to Candidate Section
10. Description of Institutional Environment
Who must complete the "Description of Institutional Environment" attachment:
The “Description of Institutional Environment” attachment is required.
Format:
Follow the page limits for the Description of Institutional Environment in the NIH Table of Page
Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Mentored CDA applicants: Describe the institution’s research and career development
opportunities related to your area(s) of interest, including the names of key faculty members and
other investigators relevant to your proposed developmental plan and capable of productive
collaboration with the candidate. Indicate how the necessary facilities and other resources will be
made available for both career enhancement and the research proposed in this application – refer
to the resources description in M.220 - R&R Other Project Information Form, Facilities and Other
Resources in your “Description of Institutional Environment” attachment. Describe opportunities
for intellectual interactions with other investigators, including courses offered, journal clubs,
seminars, and presentations.
Non-mentored CDA applicants: Describe the institution’s research and career development
opportunities related to your area(s) of interest, including the names of other faculty members
who are willing to collaborate with you. Indicate how the necessary facilities and other resources
will be made available for both career enhancement and the research proposed in this application
– refer to the resources description in M.220 - R&R Other Project Information Form, Facilities and
Other Resources in your “Description of Institutional Environment” attachment. Describe
opportunities for intellectual interactions with other investigators, including journal clubs,
seminars, and presentations.
11. Institutional Commitment to Candidate’s Research Career Development
Who must complete the "Institutional Commitment to Candidate's Research Career
Development" attachment:
The “Institutional Commitment to Candidate’s Research Career Development” attachment is
required.
M - 126

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Format:
Follow the page limits for the Institutional Commitment to Candidate’s Research Career
Development in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
The sponsoring institution must provide a document on institutional letterhead that describes its
commitment to the candidate and the candidate’s career development, independent of the receipt
of the CDA. It is also essential to document the institution's commitment to the retention,
development, and advancement of the candidate during the period of the award.
The “Institutional Commitment to Candidate’s Research Career Development” attachment should
generally document the institution’s agreement to provide adequate time, support, equipment,
facilities, and resources to the candidate for research and career development activities. See the
list below for specific items to include in the document.
In the document describing its institutional commitment, the applicant organization must:
1. Agree to release the candidate from other duties and activities so that the candidate can
devote the required percentage of time for development of a research career, as specified
by the FOA. For most K awards, commitment of at least 75 percent or nine person months
of time is required.
a. NIH and other PHS agencies use the concept of "person months" as a metric for
determining percent of effort. For more information about calculating person months,
see NIH's Frequently Asked Questions on Person Months.
2. Describe actions that will be taken to ensure that the candidate can devote the required
time to research career development (e.g., reduction of the candidate's teaching load,
committee and administrative assignments, and clinical or other professional activities for
the current academic year). If the candidate’s clinical or teaching responsibilities will be
reduced, describe how this will be accommodated (e.g., hiring additional staff, reassigning
staff, etc.).
3. Describe the candidate's academic appointment, bearing in mind that the appointment
must be full-time, and that the appointment (including all rights and privileges pertaining
to full faculty status if in an academic setting) and the continuation of salary should not be
contingent upon the receipt of this award.
4. Describe the proportion of time currently available for the candidate's research and what
the candidate's institutional responsibilities will be if an award is made.
5. Describe how the institution will provide the candidate with appropriate office and
laboratory space, equipment, and other resources (including access to clinical and/or
other research populations) to carry out the proposed Research Plan.
6. Describe how the institution will be supportive of any proposed mentor(s), other staff,
and/or collaborations with other faculty consistent with the career development plan.
Signatures:
The institutional commitment must be dated and signed by the person who is authorized to
commit the institution to the agreements and assurances listed above. In most cases, this will
be the dean or the chairman of the department. The signature must appear over the signer's
M - 127

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
name and title at the end of the statement. If the candidate will be working outside of the
applicant institution (i.e., sponsoring institution), signatures from both the
applicant/sponsoring institution and host institutions are required.
The sponsoring institution, through the submission of the application and in the institutional
commitment section, certifies that all items outlined above will be provided and that the
institution will abide by the applicable assurances and PHS policies.
Note: For applicable assurances, see the NIH Grants Policy Statement, Section 4: Public Policy
Requirements, Objectives and Other Appropriation Mandates.
12. Description of Candidate’s Contribution to Program Goals
Who must complete the "Description of Candidate’s Contribution to Program Goals" attach-
ment:
Applicants to diversity-related FOAs (e.g., diversity-related K01 and diversity-related K22s):
The “Description of Candidate’s Contribution to Program Goals” attachment is required.
All other Career Development applicants: Skip the “Description of Candidate’s Contribution to
Program Goals” attachment, as it is not required.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
The sponsoring institution must provide a document on institutional letterhead that explains how
the candidate’s participation will further the goals of the career development program to promote
diversity in health-related research. The letter should avoid revealing sensitive personally
identifiable information, such as the candidate’s specific racial/ethnic background or type of
disability.
For NIH’s Interest in Diversity, see the Notice of NIH's Interest in Diversity (NOT-OD-20-031).
Signatures:
The “Description of Candidate’s Contribution to Program Goals” attachment must be dated
and signed by an institutional official. In most cases, this will be the dean or the chairman of
the department. The signature must appear over the signer's name and title at the end of the
statement.
Other Research Plan Sections
13. Vertebrate Animals
Who must complete the “Vertebrate Animals” attachment:
Include the “Vertebrate Animals” attachment if you answered “Yes” to the question “Are
Vertebrate Animals Used?” on the M.220 - R&R Other Project Information Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
M - 128
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Do not use the Vertebrate Animals attachment to circumvent the page limits of the Research
Strategy.
Content:
If live vertebrate animals are involved in the project, address each of the following criteria:
1. Description of Procedures: Provide a concise description of the proposed procedures to
be used that involve live vertebrate animals in the work outlined in the “Research
Strategy” attachment. The description must include sufficient detail to allow evaluation of
the procedures. Identify the species, strains, ages, sex, and total numbers of animals by
species, to be used in the proposed work. If dogs or cats are proposed, provide the source
of the animals.
2. Justifications: Provide justification that the species are appropriate for the proposed
research. Explain why the research goals cannot be accomplished using an alternative
model (e.g. computational, human, invertebrate, in vitro).
3. Minimization of Pain and Distress: Describe the interventions including analgesia,
anesthesia, sedation, palliative care, and humane endpoints that will be used to minimize
discomfort, distress, pain, and injury.
Each of the criteria must be addressed. Failure to adequately address the criteria may negatively
affect the application’s impact score. In addition to the 3 criteria above, you should also:
l
Identify all project performance (or collaborating) sites and describe the proposed
research activities with vertebrate animals that will be conducted at those sites.
l
Explain when and how animals are expected to be used if plans for the use of animals
have not been finalized.
See the following pages for more information:
l
NIH’s Office of Laboratory Animal Welfare website
l
NIH's Vertebrate Animals Section Worksheet
l
NIH Grants Policy Statement, Section 4.1.1: Animal Welfare Requirements (an applicable
Animal Welfare Assurance will be required if the grantee institution does not have one)
14. Select Agent Research
Who must complete the “Select Agent Research” attachment:
Include the “Select Agent Research” attachment if your proposed activities involve the use of select
agents at any time during the proposed project period, either at the applicant organization or at
any performance site.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
For more information:
Select agents are hazardous biological agents and toxins that have been identified by HHS or the
U.S. Department of Agriculture (USDA) as having the potential to pose a severe threat to public
health and safety, to animal and plant health, or to animal and plant products. The Centers for
M - 129

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Disease Control and Prevention (CDC) and the Animal APHIS Select Agent Programs jointly
maintain a list of these agents. See the Federal Select Agent Program website.
See also the NIH Grants Policy Statement, Section 4.1.24.1: Public Health Security and Bioterrorism
Preparedness and Response Act (Select Agents).
Content:
Excluded select agents: If the activities proposed in your application involve only the use of a
strain(s) of select agents which has been excluded from the list of select agents and toxins as per
42 CFR 73.3, the select agent requirements do not apply. Use this “Select Agent Research” section
to identify the strain(s) of the select agent that will be used and note that it has been excluded
from this list. The CDC maintains a list of exclusions which is available on the Select Agents and
Toxins Exclusions website.
Applying for a select agent to be excluded: If the strain(s) is not currently excluded from the list
of select agents and toxins but you have applied or intend to apply to HHS for an exclusion from
the list, use this section to indicate the status of your request or your intent to apply for an
exclusion and provide a brief justification for the exclusion.
All applicants proposing to use select agents: Address the following three points for each site
at which select agent research will take place. Although no specific page limitation applies to this
section, be succinct.
1. Identify the select agent(s) to be used in the proposed research.
2. Provide the registration status of all entities* where select agent(s) will be used.
l
If the performance site(s) is a foreign institution, provide the name(s) of the country
or countries where select agent research will be performed.
l
*An “entity” is defined in 42 CFR 73.1 as “any government agency (Federal, State, or
local), academic institution, corporation, company, partnership, society, association,
firm, sole proprietorship, or other legal entity.”
3. Provide a description of all facilities where the select agent(s) will be used.
l
Describe the procedures that will be used to monitor possession, use and transfer of
select agent(s).
l
Describe plans for appropriate biosafety, biocontainment, and security of the select
agent(s).
l
Describe the biocontainment resources available at all performance sites.
15. Consortium/Contractual Arrangements
Who must complete the “Consortium/Contractual Arrangements” attachment:
Include the “Consortium/Contractual Arrangements” attachment if you have consortium/contracts
in your budget.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Explain the programmatic, fiscal, and administrative arrangements to be made between the
applicant organization and the consortium organization(s). If consortium/contractual activities
M - 130

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
represent a significant portion of the overall project, explain why the applicant organization, rather
than the ultimate performer of the activities, should be the grantee.
Note: The signature of the authorized organization representative in M.200 – SF 424 (R&R),
Authorized Representative signifies that the applicant and all proposed consortium participants
understand and agree to the following statement:
The appropriate programmatic and administrative personnel of each organization involved
in this grant application are aware of the agency’s consortium agreement policy and are
prepared to establish the necessary inter-organizational agreement(s) consistent with that
policy.
For more information:
Refer to the NIH Grants Policy Statement, Section 15: Consortium Agreements for more
information.
Additional Instructions for Multi-project:
Overall and Other Components: Unless otherwise specified in the FOA, you have
the option to:
l
include a single consolidated “Consortium/Contractual Arrangements”
attachment in the Overall Component, or
l
include component-specific “Consortium/Contractual Arrangements” attachment
(s) within the components that include subawards, or
l
include a “Consortium/Contractual Arrangements” attachment in the Overall
Component and include component-specific attachments within the components
that include subawards. Each filename must be unique.
16. Resource Sharing
Note: Effective for due dates on or after January 25, 2023, Data Management and
Sharing (DMS) Plans are now included in Section 11. Other Plan(s). Plans for Genomic Data
Sharing should be provided as part of the Data Management and Sharing Plan.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Sharing Model Organisms: Regardless of the amount requested, all applications where the
development of model organisms is anticipated are expected to include a description of a specific
plan for sharing and distributing unique model organisms or state why such sharing is restricted or
not possible. For more information, see the NIH Grants Policy Statement, Section 8.2.3.2: Sharing
Model Organisms.
Research Tools: NIH considers the sharing of unique research resources developed through NIH-
sponsored research an important means to enhance the value and further the advancement of the
research. When resources have been developed with NIH funds, and the associated research
findings published or provided to NIH, it is important that they be made readily available for
research purposes to qualified individuals within the scientific community. For more information,
M - 131

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
see the Research Tools Policy on the NIH Scientific Data Sharing Website and the NIH Grants
Policy Statement, Section 8.2.3: Sharing Research Resources.
For more information:
NIH considers the sharing of unique research resources developed through NIH-sponsored
research an important means to enhance the value and further the advancement of the research.
When resources have been developed with NIH funds, and the associated research findings
published or provided to NIH, it is important that they be made readily available for research
purposes to qualified individuals within the scientific community. See the NIH Grants Policy
Statement, Section 8.2.3: Sharing Research Resources.
17. Other Plan(s)
Who Must Complete This Section: Refer to the list of NIH activity codes subject to the DMS
Policy and your Funding Opportunity Announcement to determine if your application is required
to provide an attachment and address a Data Management and Sharing (DMS) Plan. Applicants
proposing to conduct research that will generate scientific data are subject to the NIH Data
Management and Sharing Policy and must attach a Data Management and Sharing (DMS) Plan.
Scientific data is defined as the recorded factual material commonly accepted in the scientific
community as of sufficient quality to validate and replicate research findings, regardless of
whether the data are used to support scholarly publications. Scientific data includes any data
needed to validate and replicate research findings. Scientific data does not include laboratory
notebooks, preliminary analyses, completed case report forms, drafts of scientific papers, plans for
future research, peer reviews, communications with colleagues, or physical objects such as
laboratory specimens.
The NIH Genomic Data Sharing Policy expects applicants seeking funding for research that
generates large-scale human or non-human genomic data to provide a plan for sharing of these
data as part of their DMS Plan.
Applicants subject to both the NIH Data Management and Sharing Policy and the NIH Genomic
Data Sharing Policy must attach a single Plan including elements for both policies. For more on
applicability of each policy, see research subject to the NIH Data Management and Sharing Policy
and the research subject to the NIH Genomic Data Sharing Policy.
Format: Attach this information as a PDF file. See NIH's Format Attachments page.
A sample format is provided on the Data Management and Sharing Plan Format Page to assist
applicants with preparation of this attachment. Do not include hyperlinks in this attachment.
Recommended not to exceed two pages.
Content: Follow the expectations of the NIH Policy for Data Management and Sharing and
address the Elements of an NIH Data Management and Sharing Plan described below.
Additional expectations: A Data Management and Sharing Plan should reflect the proposed
approach at the time the application is prepared. For some programs and data types, NIH and/or
NIH Institutes, Centers, Offices, or programs have developed additional data sharing requirements
(e.g., specifying which scientific data to share, relevant standards, repository selection, timelines)
that apply and should be reflected in a Plan. These additional requirements may be listed on NIH
Institute and Center Data Sharing Policies or in specific funding opportunity announcements. Note
that some NIH Institutes, Centers, Officers, or programs have developed additional expectations
for sharing genomic data that may be listed on NIH Institute and Center Genomic Data Sharing
Expectations or in specific funding opportunity announcements.
M - 132

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Elements of a Data Management and Sharing Plan:
Data Type: Briefly describe the scientific data to be managed, preserved, and shared,
including a general summary of the types and estimated amount of scientific data to be
generated and a description of which scientific data from the project will be preserved and
shared as well as the rationale for doing so. Briefly list the metadata, other relevant data, and
any associated documentation (e.g., study protocols and data collection instruments) that will
be made accessible to facilitate interpretation of the scientific data.
Related Tools, Software and/or Code: State whether specialized tools are needed to access or
manipulate shared scientific data to support replication or reuse, and name(s) of the needed
tool(s) and software. If specialized tools or software are needed, provide the name(s) of the
needed tool(s) and software and specify how they can be accessed.
Standards: State what common data standards will be applied to the scientific data and
associated metadata to enable interoperability of datasets and resources (e.g., data formats,
data dictionaries, data identifiers, definitions, unique identifiers, and other data
documentation), and provide the name(s) of the data standards that will be applied and
describe how these data standards will be applied to the scientific data generated by the
research proposed in this project. If applicable, indicate that no consensus standards exist.
Data Preservation, Access, and Associated Timelines: Provide plans and timelines for data
preservation and access, including the name of the repository(ies) where scientific data and
metadata arising from the project will be archived (do not include hyperlinks); how the
scientific data will be findable and identifiable, i.e., via a persistent unique identifier or other
standard indexing tools; and when (i.e., no later than time of an associated publication or end
of the performance period, whichever comes first) the scientific data will be made available to
other users (e.g., the larger research community, institutions, and/or the broader public) and
for how long. See Selecting a Data Repository on the NIH Scientific Data Sharing website.
Access, Distribution, or Reuse Considerations: NIH expects that in drafting Plans, researchers
maximize the appropriate sharing of scientific data generated from NIH-funded or conducted
research, consistent with privacy, security, informed consent, and proprietary issues. Describe
and justify any applicable factors affecting subsequent access, distribution, or reuse of
scientific data related to informed consent, privacy and confidentiality protections, any
restrictions imposed by federal, Tribal, or state laws, regulations, or policies, or existing or
anticipated agreements, or any other considerations that may limit the extent of data sharing.
See Frequently Asked Questions for examples of justifiable reasons for limiting sharing of
data. State whether access to the scientific data will be controlled (i.e., made available by a
data repository only after approval).
Genomic Data Sharing Policy: For proposed research subject to the GDS Policy, state
whether data, including genomic summary results, will be made available through controlled
or unrestricted access; see instructions for describing Genomic Summary Results in Data
Management and Sharing Plans.
If generating scientific data derived from humans, describe how the privacy, rights, and
confidentiality of human research participants will be protected (e.g., through de-
identification, Certificates of Confidentiality, and other protective measures). See NIH’s
Scientific Data Sharing page for additional information on protecting human research
participant privacy when sharing data.
Genomic Data Sharing Policy: For proposed research generating human genomic data
within the scope of the GDS Policy, applicants should complete the Data Management and
M - 133

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Sharing Plan anticipating sharing according to the assurances of the Institutional
Certification.
If there is any element of the Institutional Certification that the institution (in consultation
with the IRB) has determined cannot be met, please state which element and provide a
detailed explanation for why the element cannot be met. In such cases, the data management
and sharing plan should describe how genomic data will be shared to the maximal extent
possible (for example, sharing data in a summary format).
Oversight of Data Management and Sharing: Describe how compliance with the Plan will be
monitored and managed, frequency of oversight, and by whom at the applicant institution
(e.g., titles, roles).
For more information on developing a Data Management and Sharing Plan, see Writing a
Data Management and Sharing Plan on the NIH Scientific Data Sharing website.
For more information on the DMS Policy, including expectations for data management and
sharing, protecting research participant privacy, and identifying data repositories, see the NIH Data
Management and Sharing Policy on the NIH Scientific Data Sharing website and the NIH Grants
Policy Statement, Section 8.2.3.1: Data Sharing Policy. See also Frequently Asked Questions for
additional information on the DMS Policy on these and other topics.
For more information on the GDS Policy see the NIH Genomic Data Sharing Policy on the NIH
Scientific Data Sharing website and the NIH Grants Policy Statement, Section 8.2.3.3: Genomic
Data Sharing (GDS) Policy/ Policy for Genome-Wide Association Studies (GWAS)
Additional Instructions for Multi-project:
Overall Component Include a single consolidated “Data Management and
Sharing Plan” in the Overall Component.
Other Components: Do not include a “Data Management and Sharing Plan”
within other components. Any component-specific information should be described
within the overall “Data Management and Sharing Plan” attachment in the Overall
Component.
18. Authentication of Key Biological and/or Chemical Resources
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
If applicable to the proposed science, briefly describe methods to ensure the identity and validity
of key biological and/or chemical resources used in the proposed studies. A maximum of one page
is suggested.
More information:
Key biological and/or chemical resources are characterized as follows:
l
Key biological and/or chemical resources may or may not have been generated with NIH
funds and: 1) may differ from laboratory to laboratory or over time; 2) may have qualities
and/or qualifications that could influence the research data; and 3) are integral to the
proposed research. These include, but are not limited to, cell lines, specialty chemicals,
M - 134

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
antibodies, and other biologics.
l
Standard laboratory reagents that are not expected to vary do not need to be included in
the plan. Examples are buffers and other common biologicals or chemicals.
l
See NIH's page on Rigor and Reproducibility for more information.
Appendix
19. Appendix
Refer to the FOA to determine whether there are any special appendix instructions for your
application. See the updated NIH Guide Notice on the Appendix Policy.
Additional Instructions for Multi-project:
Overall and Other Components: The "Appendix” attachment is optional.
Format:
A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 allowable appendix
attachments are needed, combine the remaining information into attachment #10.
Use filenames for attachments that are descriptive of the content.
A summary sheet listing all of the items included in the Appendix is encouraged but not required.
When including a summary sheet, it should be included in the first appendix attachment.
Content:
The only allowable appendix materials are:
l
Blank data collection forms, blank survey forms, and blank questionnaire forms - or
screenshots thereof
l
Simple lists of interview questions
Note: In your blank forms and lists, do not include items such as: data, data
compilations, lists of variables or acronyms, data analyses, publications, manuals,
instructions, descriptions or drawings/figures/diagrams of data collection methods or
machines/devices.
l
Blank informed consent/assent forms
l
Other items only if they are specified in the FOA as allowable appendix materials
No other items are allowed in the Appendix. Simply relocating disallowed materials to other parts
of the application will result in a noncompliant application.
Some FOAs may have different instructions for the Appendix. Always follow the instructions in
your FOA if they conflict with these instructions.
Note: Applications will be withdrawn and not reviewed if they do not follow the appendix
requirements in these instructions or in your FOA.
Information that expands upon or complements information provided in any section of the
application – even if it is not required for the review – is not allowed in the Appendix unless it is
M - 135

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
listed in the allowed appendix materials above or in your FOA. For example, do not include
material transfer agreements (MTA) in the appendix unless otherwise specified in the FOA.
For more information:
l
The NIH Guide Notice on Reminder: NIH Applications Must Be Complete and Compliant With
NIH Policy and Application Instructions At Time of Submission.
l
Failure of reviewers to address non-required appendix materials in their reviews is not an
acceptable basis for an appeal of initial peer review. For more information, see the NIH Grants
Policy Statement, Section 2.4.2: Appeals of Initial Scientific Review.
l
Appendix Policy Frequently Asked Questions
Citizenship
Information on Citizenship Requirements for CDA Applicants:
The candidate must be a citizen or non-citizen national of the United States or its possessions and
territories, or must have been lawfully admitted to the United States for permanent residence by
the time of award EXCEPT if any of the following apply:
l
candidate is applying to the K99/R00 award program;
l
candidate is applying to the K43 award program; or
l
the FOA specifies otherwise.
Note for permanent residents: Before an award is issued, a permanent resident will be required
to submit a notarized statement that the candidate holds a current and valid Permanent Resident
Card or some other valid verification from the U.S. Immigration and Naturalization Service of legal
admission to the U.S. as a permanent resident.
Note for candidates whose citizenship status changes or is expected to change: For those
career development award programs that require candidates to be U.S. citizens or permanent
residents, an individual who has applied for permanent residence and expects to have obtained
such status prior to the time award may submit an application recognizing that no award will be
made until legal verification of permanent resident status is provided. If a candidate’s citizenship
status changes after submission of the application, the new status should be reported in the
candidate’s Personal Profile in the eRA Commons.
Note on K99/R00 applicants on temporary visas: It is the responsibility of the applicant
organization to determine and document in the application that the candidate’s visa will allow him
or her to remain in the U.S. long enough to complete the phase of the award (e.g., K99 or R00)
covered by the application. Information may be requested by the NIH or another PHS Agency prior
to issuance of an award as a Just-in-Time submission.
Check the applicable boxes for the following questions:
20. U.S. Citizen or Non-Citizen National?
Check “Yes” if the candidate is either a U.S. Citizen or a Non-Citizen national; otherwise check “No.”
M - 136

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.410 - PHS 398 Career Development Award Supplemental Form
Non-Citizen nationals are people who, although not citizens of the United States, owe permanent
allegiance to the United States. They generally are people born in outlying possessions of the
United States (e.g., American Samoa and Swains Island).
If no, select most appropriate Non-U.S. Citizen option:
Please select the most appropriate response from the options provided.
With a Permanent U.S. Resident Visa:
Check this box if the candidate has been lawfully admitted for permanent residence (i.e., is in
the possession of a current and valid Permanent Resident Card or other legal verification of
such status). A notarized statement will be required as part of the pre-award process.
With a Temporary U.S. Visa:
Check this box if the candidate currently holds a temporary U.S visa. This box is applicable
only to specific programs that do not require U.S. citizenship or permanent residency (e.g.,
K99/R00).
Not Residing in the U.S.:
Check this box if the candidate is a citizen of a country other than the U.S. and plans to
pursue career development outside of the U.S. This box is applicable only to specific
programs (e.g., K43).
If you are a non-U.S. citizen with a temporary visa applying for an award that requires per-
manent residency status, and expect to be granted a permanent resident visa by the start
date of the award, check here:
Check this box to indicate that permanent resident status is pending (i.e., if the candidate is not a
U.S citizen but has applied for permanent residence and expects to hold a permanent resident visa
by the earliest possible start date of the award). A notarized statement will be required as a part of
the pre-award process. The statement must show that a licensed notary has seen the career
development applicant’s valid Permanent Resident Card (USCIS Form I-551) or other valid
verification from the U.S. Immigration and Naturalization Service of legal admission to the U.S.
M - 137

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
M.420 - PHS 398 Research Training
Program Plan Form
The PHS 398 Research Training Program Plan Form is
used only for Training applications and Multi-project
applications with an "NRSA Training" Component.
This form includes fields to upload several attachments
including the Program Plan, Faculty Biosketches, and
Data Tables.
The attachments in this form, together with the rest of
your application, should include sufficient information
needed for evaluation of the training plan, independent
of any other documents (e.g., previous application). Be
specific and informative, and avoid redundancies.
View larger image
Quick Links
Introduction
1. Introduction to Application (for Resubmission and Revision applications)
Training Program Section
2. Program Plan
3. Plan for Instruction in the Responsible Conduct of Research
4. Plan for Instruction in Methods for Enhancing Reproducibility
5. Multiple PD/PI Leadership Plan (if applicable)
6. Progress Report (for Renewal applications)
Faculty, Trainees, and Training Record Section
7. Participating Faculty Biosketches
8. Letters of Support
9. Data Tables
Other Training Program Section
10. Vertebrate Animals
11. Select Agent Research
12. Consortium/Contractual Arrangements
13. Other Plan(s)
Appendix
M - 138

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
14. Appendix
Who should use the PHS398 Research Training Program Plan Form:
Use the PHS 398 Research Training Program Plan Form only if you are submitting a training application
or a multi-project application that has an "NRSA Training" Component.
Read all the instructions in the FOA before completing this section to ensure that your application
meets all IC-specific criteria.
Note on required tables: The instructions for the required Data Tables (1-8) are located on the NIH's
Data Tables page. Please read the “Introduction to Data Tables” before beginning to prepare your data
tables. The Introduction to Data Tables includes important definitions that should be used consistently
both in the "Data Tables" attachment of your application and in all other parts of the application. The
Data Tables must be included in the "Data Tables" attachment to avoid being counted against the page
limits of other attachments.
Note on non-required tables: Additional tables (i.e., those that are generated by the applicant or not
required by the FOA) should be identified by letter, rather than number, to avoid confusion with the
sequentially numbered required tables.
Applicants must follow all policies and requirements related to formatting, page limits, and
proprietary information. See the following pages for more information:
l
Format Attachments
l
Page Limits
l
NIH Grants Policy Statement, Section 2.3.11.2: Confidentiality of Information
l
NIH Grants Policy Statement, Section 2.3.11.2.2: The Freedom of Information Act
Introduction
1. Introduction to Application (for Resubmission and Revision applications)
Who must complete the “Introduction to Application” attachment:
An "Introduction to Application" attachment is required only if the type of application is
resubmission or revision or if the FOA specifies that one is needed. An introduction is not allowed
for new or renewal applications.
Descriptions of different types of applications are listed here: NIH Types of Applications.
Format:
Follow the page limits for the Introduction in the NIH Table of Page Limits unless otherwise
specified in the FOA. Note that page limits for the Introduction may differ based on the type of
application (i.e., resubmission or revision).
Attach this information as a PDF file. See NIH's Format Attachments page.
M - 139

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
Content:
Resubmission Applications: See specific instructions on the content of the Introduction on the
NIH's Resubmission Applications page.
Note: For resubmission applications changing from a single PD/PI to multiple PD/PIs,
changing the number or makeup of the multiple PD/PIs, the applicant must provide a
rationale for the change in the introduction and include the required Multiple PD/PI
Leadership Plan. A rationale for a change from a multiple PD/PI to a single PD/PI application
must also be provided in the introduction.
Competing Revision Applications: See specific instructions on the content of the Introduction
on the NIH's Competing Revisions page.
Additional Instructions for Multi-project:
Other Components: The "Introduction” attachment is optional for resubmissions
and revisions applications. Although the “Introduction” attachment is optional, you
may get a system warning if there is no attachment.
Training Program Section
2. Program Plan
Who must complete the “Program Plan” attachment:
The “Program Plan” attachment is required.
Format:
Follow the page limits for the Program Plan in the NIH Table of Page Limits unless otherwise
specified in the FOA. The Program Plan (including sections "A. Background;" "B. Program Plan;"
and "C. Recruitment Plan to Enhance Diversity," when applicable) must fit within the Program Plan
page limit unless otherwise specified in the FOA.
Note that Data Tables may be referred to or summarized in this section; however, the actual tables
are not to be included in this attachment.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Organize the Program Plan attachment in the specified order and use the instructions provided
below unless otherwise specified in the FOA. Start each section with the appropriate heading –
Background, Program Plan, and Recruitment Plan to Enhance Diversity. In addition, start each
subsection of the Program Plan with the appropriate subheading.
Check the FOA and the instructions for the Data Tables to determine which tables should be
included in the application and discussed in the Program Plan subsection.
A. Background
Provide the rationale for the proposed research training program, the relevant background history,
and the need for the proposed research training.
M - 140

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
Indicate how the proposed program relates to current training activities at the applicant
institution.
Summarize the research training activities of the major participating unit(s) and department(s)
represented in the proposed program.
If required, complete Tables 1-3 (these tables will be included in the Data Tables attachment), and
summarize the data here using the guidance below. In your narrative, refer to specific tables as
applicable.
Table 1. Census of Participating Departments and Interdepartmental Programs:
Describe the organization of the proposed training program, the participating departments
and interdepartmental programs, and the extent to which faculty, graduate students, and/or
postdoctorates from those departments/interdepartmental programs participate in the
programmatic activities to be supported by the training grant.
Table 2. Participating Faculty Members: Describe the distribution of participating faculty
by academic rank, department or interdepartmental program and areas of research
emphasis. Describe the rationale for the faculty selected to participate in the training grant.
Analyze the data in terms of the overall experience of the faculty in training predoctorates
and/or postdoctorates. Comment on the inclusion of faculty whose mentoring records may
suggest limited, recent training experience at either training level (predoctoral or
postdoctoral).
Table 3. Federal Institutional Research Training Grant and Related Support Available
to Participating Faculty Members: Summarize the level of research training support at the
institution. Comment on instances where the tabular data indicate that there may be
substantial overlap of participating faculty.
B. Program Plan
Note: Applicants for institutional career development awards (e.g., K12) must complete a Research
Career Development Program Plan instead of the Training Program Plan. Refer to specific
instructions in the FOA.
a. Program Administration
Program Director information: Describe the program director's qualifications for providing
leadership of the program, including relevant scientific background, current research areas, and
experience in research training. Indicate the program director's percent effort in the proposed
program.
Administrative information: Describe the administrative structure of the program and the
distribution of responsibilities within it, including the means by which the program director will
obtain continuing advice with respect to the operation of the program.
Special Instructions for Multiple PD/PI: If multiple PD/PIs are proposed, explain in this
section your rationale for how this will facilitate program administration. In addition, you must
complete the Multiple PD/PI Leadership Plan attachment in this form.
Renewal Applications: For renewal applications changing from a single PD/PI to multiple
PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant must provide a
rationale for the change in the program plan and include the required Multiple PD/PI
Leadership Plan. A rationale for a change from a multiple PD/PI to a single PD/PI application
must also be provided in the program plan.
M - 141

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
b. Program Faculty
Referring to the data presented in Table 2. Participating Faculty Members, describe each faculty
member's research that is relevant to the program and indicate how trainees will participate in the
research. Provide information on the extent to which participating faculty members have
cooperated, interacted, and collaborated in the past, including joint publications and joint
sponsorship of student research.
Use this section to document the ability of the faculty to support the research activities of the
proposed trainees, the training record of the participating faculty members, and the success of
their trainees in generating publishable research results. For any proposed participating faculty
(i.e., program faculty) members lacking research training experience, describe a plan to ensure that
they will successfully guide trainees. Describe the criteria used to appoint and remove faculty as
program faculty and to evaluate their participation.
If required, complete Tables 4-5 (these Tables will be included in the Data Tables attachment), and
summarize the data here using the guidance below. In your narrative, refer to specific tables, as
applicable.
Table 4. Research Support of Participating Faculty Members: Analyze the data in terms
of total and average grant support. Additionally, comment on the inclusion of faculty without
research grant support and explain how the research of trainees who may work with these
faculty members would be supported.
Table 5A-C. Publications of Those in Training: Summarize these data, including, for
example, the average number of publications, and how many students have published their
work. For pre- and postdoctoral training programs, indicate how many trainees are published
as first author, and how many completed their doctoral or postdoctoral training without any
first-author publication.
Note for New Applications: List publications for students and/or postdoctorates who are
representative of those who would be appointed if the grant is awarded.
c. Proposed Training
Describe the proposed training program. Indicate the training level(s) and number of trainees, the
academic and research background needed to pursue the proposed training, and, as appropriate,
plans to accommodate differences in preparation among trainees. For postdoctoral trainees,
indicate the proposed distribution by degree (e.g., M.D., Ph.D.). Describe course work, research
opportunities and the extent to which trainees will participate directly in research, activities
designed to develop technical and/or professional skills, and the duration of training, i.e., usual
period of time required to complete the training offered.
Describe how the program and faculty will provide training in scientific reasoning, rigorous
research design, relevant experimental methods, relevant quantitative and data science
approaches, and data analysis and interpretation, appropriate to the level and prior preparation of
the trainees.
For multi-disciplinary and/or multi-departmental programs, indicate how the individual
disciplinary and/or departmental components of the program are integrated and coordinated and
how they will relate to an individual trainee's experience.
For training programs that emphasize research training for clinicians, describe the interactions with
basic science departments and scientists. Include plans for ensuring that the training of these
individuals will provide a substantive foundation for a competitive research career. Generally, a
minimum of 2 years of research training is expected for all postdoctoral trainees with health
M - 142

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
professional degrees. Describe fully any trainee’s access to and responsibility for patients,
including time commitment.
Training programs that anticipate offering trainees opportunities to be involved in human subjects
research funded by other research grants may include a brief description of those opportunities in
this section, although such a description is not required.
Provide representative examples of programs for individual trainees. Include curricula, degree
requirements, didactic courses, laboratory experiences, qualifying examinations, and other training
activities, such as seminars, journal clubs, etc. Describe how the mentor and research areas are
chosen, how each trainee's program will be guided, and how the trainee's performance will be
monitored and evaluated. Include detailed mentoring plans as appropriate.
d. Training Program Evaluation
Describe an evaluation plan to review and determine the quality and effectiveness of the training
program. This should include plans to obtain feedback from current and former trainees to help
identify weaknesses in the training program and to provide suggestions for program
improvements. Specified evaluation metrics should be tied to the goals of the program. In
addition, describe plans for assessing the career development and progression of trainees,
including publications, degree completion, and post-training positions.
Renewal Applications: Discuss evaluation results, and indicate whether the program has been
modified as a result.
e. Trainee Candidates
Describe, in general terms, the size and qualifications of the pool of trainee candidates, including
information about the types of prior clinical and research training and the career level required for
the program. Describe specific plans to recruit candidates and explain how these plans will be
implemented (see also "Section C. Recruitment Plan to Enhance Diversity" within the Program
Plan). Describe the nomination and selection process to be used to select candidates who will be
offered admission to the program and criteria for trainees’ reappointment to the program.
If required, complete Tables 6A and/or 6B (these Tables will be included in the Data Tables
attachment), and summarize the data here using the guidance below. In your narrative, refer to
specific tables as applicable.
Tables 6A and/or 6B. Applicants, Entrants, and their Characteristics for the Past Five
Years (Predoctoral and Postdoctoral). Summarize the data in terms of the overall numbers
of potential trainees, their credentials, their characteristics, their eligibility for support, and
enrollment trends.
f. Institutional Environment and Commitment to Training
Include information in the application that documents the support and commitment of the
applicant organization and participating units and departments to the goals of the proposed
program. This could include, for example, space, shared laboratory facilities and equipment, funds
for curriculum development, release time for the PD/PI and participating faculty, support for
additional trainees in the program, or any other creative ways to improve the environment for the
establishment and growth of the research training program.
Include a signed letter, on institutional letterhead, that describes the applicant organization’s
commitment to the planned program (see instructions in the Letters of Support section).
Institutions with ongoing research training, student development, or career development
programs that receive external funding should explain what distinguishes the proposed program
M - 143

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
from existing ones at the same trainee level; how the programs will synergize, if applicable;
whether trainees are expected to transition from one support program to another; and how the
training faculty, pool of potential trainees, and resources are sufficiently robust to support the
proposed program in addition to existing ones.
g. Qualifications of Trainee Candidates and Admissions and Completion Records
Describe the ability of the participating departments/programs to recruit and retain trainees
through the completion of their training, the selectivity of the admissions process, and the success
of the departments/programs in recruiting individuals from diverse backgrounds (see also Section
C. Recruitment Plan to Enhance Diversity within the Program Plan).
Discuss the quality and depth of the applicant pools, including both training-grant eligible and
non-training-grant eligible individuals, the competitiveness of the program, and the characteristics
of current program participants, referring to the data in Tables 6A and/or 6B, as applicable.
Use all of this information to justify the number of positions requested.
If required, complete Tables 7-8 (these Tables will be included in the Data Tables attachment) and
summarize the data using the guidance below. In your narrative, refer to specific tables as
applicable.
Table 7. Appointments to the Training Grant for Each Year of the Current Project
Period: Describe the utilization of awarded training positions. If any trainee positions were
not filled, if any trainees terminated early, or if the distribution of appointed positions differs
from the distribution of awarded positions, provide an explanation.
Table 8A-D. Program Outcomes: Referring to relevant components of Table 8 (e.g. 8A, 8B,
8C and/or 8D, as appropriate), describe how training positions are used (i.e., distribution by
mentor, year in program, years of support per trainee), and the success of the program in
achieving its training objectives. For those who have completed their training, describe the
extent of their current involvement in research, including research grant support received
subsequent to completion of the training program.
Renewal applications: Discuss the selectivity of appointments to the training grant, and if
any postdoctoral trainee with a health professional degree was appointed to a Kirschstein-
NRSA training grant for less than 2 years of research training, explain why.
C. Recruitment Plan to Enhance Diversity
Who must complete the “Recruitment Plan to Enhance Diversity:”
A Recruitment Plan to Enhance Diversity is required for all training grant activity codes except T34,
T36, U2R, and all D-series activity codes. All other applications without a Recruitment Plan to
Enhance Diversity will be considered incomplete and will not be reviewed.
Content:
History and Achievements
Describe efforts to recruit individuals from underrepresented groups, including Diversity
Groups A, B, and C, as potential candidates for the existing training program. Refer to the
Notice of NIH's Interest in Diversity (NOT-OD-20-031) for the descriptions of Diversity
Groups. As applicable, refer to the data presented in Tables 6 and 7. Use these data to
document the program’s past record of recruiting trainees who are underrepresented and to
provide information on their support.
M - 144
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
Proposed Plans
Describe steps to be taken during the proposed award period to identify and recruit graduate
students and postdoctorates from Diversity Groups A, B, and C. Refer to the Notice of NIH's
Interest in Diversity (NOT-OD-20-031) for the descriptions of Diversity Groups. Consider the
success and/or failures of recruitment strategies used in the past. In particular, describe the
specific efforts to be undertaken by the training program and how these might relate to the
recruitment efforts of the medical school, graduate school, and/or the university at large. In
most cases, centralized institutional efforts alone will not satisfy the requirement to recruit
individuals from underrepresented groups, and training grant faculty are expected to be
actively involved in recruitment efforts.
New Applications: Include a description of plans to enhance recruitment, including the strategies
that will be used to enhance the recruitment of potential trainees from underrepresented groups.
Renewal Applications: Include a detailed account of experiences in recruiting individuals from
underrepresented groups during the previous funding period, including successful and
unsuccessful recruitment strategies. Information should be included on how the proposed plan
reflects the program’s past experiences in recruiting individuals from underrepresented groups.
For more information:
Refer to the Notice of NIH's Interest in Diversity (NOT-OD-20-031).
3. Plan for Instruction in the Responsible Conduct of Research
Who must complete the “Plan for Instruction in the Responsible Conduct of Research”
attachment:
A “Plan for Instruction in the Responsible Conduct of Research (RCR)” attachment is required for all
training grant activity codes except T36, unless otherwise noted in the FOA. Applications lacking a
Plan for Instruction in RCR will not be reviewed.
Format:
Follow the page limits for the Plan for Instruction in the Responsible Conduct of Research in the
NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
The plan must address the five required instructional components outlined in the NIH Policy on
Instruction in RCR, as more fully described in the NIH Grants Policy Statement, Section 11.3.3.5:
Training in the Responsible Conduct of Research:
1. Format: Describe the required format of instruction, i.e., face-to-face lectures,
coursework, and/or real-time discussion groups. A plan with only on-line instruction is not
acceptable.
2. Subject Matter: Describe the breadth of subject matter, e.g., conflict of interest,
authorship, data management, human subjects and animal use, laboratory safety,
research misconduct, and research ethics.
3. Faculty Participation: Describe the roles of mentor(s) and other faculty involvement in
the instruction.
M - 145

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
4. Duration of Instruction: Describe the total number of contact hours of instruction.
5. Frequency of Instruction: Instruction must occur during each career stage and at least
once every four years. Document any prior instruction during the applicant’s current
career stage, including the inclusive dates instruction was last completed.
The plan must also describe how participation in RCR instruction will be monitored.
Renewal Applications: Describe any changes in formal instruction over the past project period
and plans for the future that address any weaknesses in the current RCR instruction. All training
faculty who served as course directors, speakers, lecturers, and/or discussion leaders during the
past project period must be named in the application.
For more information:
See the NIH Grants Policy Statement, Section 11.3.3.5: Training in the Responsible Conduct of
Research.
4. Plan for Instruction in Methods for Enhancing Reproducibility
Who must complete the “Plan for Instruction in Methods for Enhancing Reproducibility”
attachment:
A “Plan for Instruction in Methods for Enhancing Reproducibility” attachment is required for all
training grant activity codes except D71, unless otherwise noted in the FOA. Applications lacking a
Plan for Instruction in Methods for Enhancing Reproducibility will not be reviewed.
Format:
Follow the page limits for the Plan for Instruction in Methods for Enhancing Reproducibility in the
NIHTable of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
The plan must describe how trainees will be instructed in principles important for enhancing
research reproducibility. These principles include, at a minimum, the following:
l
evaluation of the foundational research underlying a project (i.e., the rigor of the prior
research);
l
rigorous experimental design and data interpretation;
l
consideration of relevant biological variables such as sex;
l
authentication of key biological and/or chemical resources; and
l
transparency in reporting.
Include a description of how instructional strategies will be integrated into the overall training
program at multiple stages of trainee development and in a variety of formats and contexts.
Describe how program faculty will reiterate and augment key elements of methods for enhancing
reproducibility in the context of trainees’ research projects.
M - 146

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
5. Multiple PD/PI Leadership Plan (if applicable)
Who must complete the “Multiple PD/PI Leadership Plan” attachment:
Any applicant who designates multiple PD/PIs (on the M.240 - R&R Senior/Key Person Profile
(Expanded) Form) must include a Multiple PD/PI Leadership Plan. For applications designating
multiple PD/PIs, all such individuals must be assigned the PD/PI role on the M.240 - R&R
Senior/Key Profile (Expanded) Form, even those at organizations other than the applicant
organization.
Do not submit a leadership plan if you are not submitting a multiple PD/PI application.
Additional Instructions for Multi-project:
Overall Component: The “Multiple PD/PI Leadership Plan” attachment is required
only in the Overall Component.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
The emphasis in a training grant's Multiple PD/PI Leadership Plan should be on how multiple
PD/PIs will benefit the program and the trainees. A single PD/PI must be designated as Contact
PD/PI (in M.200 - SF 424 (R&R) Form, PD/PI Contact Information) for the purpose of
communicating with the NIH, although other individuals may contact the NIH on behalf of the
Contact PD/PI when necessary. Because training programs are intended to be coherent, NIH will
not allocate the budget or training positions between multiple PD/PIs. A single award will be
made. Multiple PD/PI plans should include reasonable numbers of PD/PIs and each should be
included for a specific and clearly stated purpose.
A rationale for choosing a multiple PD/PI approach should be described. The governance and
organizational structure of the leadership team and the training program should be described,
including communication plans, processes for making decisions, and procedures for resolving
conflicts. The roles and administrative, technical, and other responsibilities for the training
program should be delineated for the PD/PIs and other collaborators.
Resubmission Applications: For resubmission applications changing from a single PD/PI
to multiple PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant
must provide a rationale for the change in the introduction and include the required Multiple
PD/PI Leadership Plan.
Renewal Applications: For renewal applications changing from a single PD/PI to multiple
PD/PIs, changing the number or makeup of the multiple PD/PIs, the applicant must provide a
rationale for the change in the program plan and include the required Multiple PD/PI
Leadership Plan.
For more information:
For background information on the multiple-PD/PI initiative, see NIH's Multiple Principal
Investigators page.
M - 147

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
6. Progress Report (for Renewal applications)
Who must complete the “Progress Report” attachment:
A “Progress Report” attachment is required only if the type of application is renewal.
Format:
Follow the page limits given below, unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Organize the Progress Report according to the specified sections. Start each section with the
appropriate heading – Program Overview or Progress of Those Appointed to the Grant.
Program Overview (Page limit: 5 pages)
Provide an overview of accomplishments and progress achieved in the period since the last
competitive review. Focus on elements specific to the training program (rather than on
opportunities generally available in the institution’s other departments or other programs).
Describe how the funds provided under Training Related Expenses were used to benefit the
program.
List any workshops or seminars sponsored by the program. Include the workshop/seminar titles,
speakers, and relevance to the theme and training objectives of the program.
Indicate whether the training program uses Individual Development Plans (IDPs). If so, describe
how IDPs were used in this reporting period to help manage the trainees’/scholars’ training and
career development.
Note: Do not include actual IDPs or blank IDP forms.
Note for AHRQ trainees: Neither IDPs nor information about IDPs is required.
You may refer to information that is included elsewhere in the application, such as the Program
Plan or outcomes described in the Training Data Tables, but do not repeat that information in the
Progress Report.
Progress of Those Appointed to the Grant (Page limit: 1 page per appointee)
For each trainee or scholar appointed to the grant in the period covered since the last competitive
review, provide a summary of his or her training and progress, including the following information,
as applicable:
l
Degrees working toward or received;
l
Mentor(s);
l
Description of the trainee/scholar’s research project and progress;
l
Career development activities (e.g., individualized coursework or workshops attended);
l
Conference presentations;
l
A description of the trainee’s contribution to any planned or published papers resulting
from research conducted while supported by this award (e.g., designed or conducted
experiment, analyzed data, drafted paper); and
M - 148

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
l
Honors, awards, fellowships, and any other support received during the period of training.
Note: Support before and after the appointment is reported in the Data Tables and
should not be reported here.
Do not include the following, either in the Progress Report or elsewhere in the application
(including the Appendix), unless otherwise specified in the FOA:
l
Biosketches of current or former trainees/scholars;
l
Any sensitive personally identifiable information, such as photographs or any other
individual demographic information;
l
Actual IDPs or blank IDP forms;
l
Promotional material for workshops, seminars, or other events (flyers, agendas, etc.);
l
Course syllabi; and
l
Program brochures.
Applications that include any of these materials will be withdrawn and not reviewed.
Note: A My Bibliography report of publications arising from work conducted by trainees while
supported by the training grant is not required in the application. However, it will be collected in
the Interim Final Research Performance Progress Report.
Additional Instructions for Multi-project:
Overall and Other Components: If you include a “Progress Report Publication List”
attachment, you can include it in either the Overall Component or within the Other
Component, but do not attach the same information in multiple locations.
Faculty, Trainees,andTraining Record Section
7. Participating Faculty Biosketches
Format:
Combine all participating faculty biosketches into a single PDF and attach this information here.
Follow the attachment guidelines on NIH's Format Attachments page.
Content:
Faculty biosketches for participating faculty must follow the instructions for a biographical sketch
(refer to M.240 - Senior/Key Person Profile (Expanded) Form) with the following exception: a
personal statement, while encouraged, is not required.
Please note that the biosketches of the PD/PI and any other senior/key personnel (e.g., co-
directors, if applicable, and program staff) should not be included here, but they should instead be
included in the M.240 - R&R Senior/Key Person Profile (Expanded) Form.
M - 149

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
8. Letters of Support
Format:
Combine all Letters of Support into a single PDF file and attach this information here. Do not place
these letters in the Appendix. Follow the attachment guidelines on NIH's Format Attachments
page. Use of hyperlinks and URLs in Letters of Support is not allowed unless specified in the
funding opportunity announcement.
Content:
Attach letters here from:
l
Consultants, if applicable. Letters should include rate/charge for consulting services and
confirm their role(s) in the project.
l
Senior Administration Officials. This letter should be a signed letter on institutional
letterhead, and it should describe the applicant institution’s commitment to the planned
program.
l
A President, Provost, Dean, Department Chair, or other key institutional leader with institution-
wide responsibilities. This letter should be a signed letter on institutional letterhead, and it
should describe and acknowledge institutional commitment to the following areas:
l
Ensuring that proper policies, procedures, and oversight are in place to prevent
discriminatory harassment and other discriminatory practices;
l
Responding appropriately to allegations of discriminatory practices, including any
required notifications to the HHS Office of Civil Rights; and
l
Adopting and following institutional procedure for requesting NIH prior approval of a
change in the status of the Program Director/Principal Investigator (PD/PI) or other
senior/key personnel if administrative or disciplinary action is taken that impacts the
ability of the PD/PI or other key personnel to continue his/her role on the NIH award
as described in the training grant application.
Check the FOA (particularly for non-NRSA programs) to determine whether any additional
program-specific letters of support are required.
For more information:
Notice of Clarification Regarding Harassment and Discrimination Protections in NIH Training
Applications
NIHGrants Policy Statement, Section 4.1.2: Civil Rights Protections
NIHGrants Policy Statement, Section 8.1.2.6: Change in Status, Including Absence of PD/PI and
Other Senior/Key Personnel Named in the NOA.
9. Data Tables
Format:
The information provided in the required data tables (Data Tables 1-8 described below) will not be
counted toward the page limitation. These tables should be numbered consecutively and titled as
instructed. Start each numbered table on a new page.
M - 150

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
Bookmark each table separately in the PDF attachment. Many PDF generators will automatically
create bookmarks from text formatted using predefined Heading styles in Word.
Combine all Data Tables into a single PDF file and attach it here. See NIH's Format Attachments
page.
Content:
Instructions for Data Tables 1-8 are located on NIH's Data Tables page. These instructions include
an Introduction to the Data Tables that provides instructions applicable to all tables, specific
instructions for each table, and Sample Data Tables. The sample data tables illustrate the kind of
data to include in each table for training grant applications.
If not using the Extramural Trainee Reporting and Career Tracking (xTRACT) system to prepare
data tables, be sure to choose the Instruction and Blank Data Table set that correspond to both the
type of application you are submitting (e.g., new application, renewal or revision application) and
the kind of training to be provided (e.g., predoctoral only, postdoctoral only, pre and postdoctoral
mixed, etc.).
Other Training Program Section
10. Vertebrate Animals
Who must complete the “Vertebrate Animals” attachment:
Include a “Vertebrate Animals” attachment if you answered “Yes” to the question “Are Vertebrate
Animals Used?” on the M.220 - R&R Other Project Information Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Do not use the Vertebrate Animals attachment to circumvent the page limits of the Program Plan.
Content:
Trainee Participation Only in Research Involving Vertebrate Animals that is Part of Other
Research Project Grants: Describe how the institution will ensure that trainees participate only in
IACUC-approved vertebrate animal research if the following two conditions apply:
l
the training program uses live vertebrate animals only as part of other research project
grants, and
l
the training grant does not support the purchase, use, or husbandry of live vertebrate
animals.
Independent Trainee Research Involving Vertebrate Animals: In training programs where
trainees will design and conduct their own independent vertebrate animal research, follow the
instructions below:
Address each of the following criteria:
1. Description of Procedures: Provide a concise description of the proposed procedures to
be used that involve live vertebrate animals in the work outlined in the “Program Plan”
attachment. The description must include sufficient detail to allow evaluation of the
procedures. Identify the species, strains, ages, sex, and total numbers of animals by
M - 151

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
species, to be used in the proposed work. If dogs or cats are proposed, provide the source
of the animals.
2. Justifications: Provide justification that the species are appropriate for the proposed
research. Explain why the research goals cannot be accomplished using an alternative
model (e.g. computational, human, invertebrate, in vitro).
3. Minimization of Pain and Distress: Describe the interventions, including analgesia,
anesthesia, sedation, palliative care, and humane endpoints, that will be used to minimize
discomfort, distress, pain, and injury.
Each of the criteria must be addressed. Failure to adequately address the criteria may negatively
affect the application's impact score. In addition to the three criteria above, you should also:
l
Identify all project performance (or collaborating) sites and describe the proposed
research activities with vertebrate animals that will be conducted at those sites.
l
Explain when and how animals are expected to be used if plans for the use of animals
have not been finalized.
See the following pages for more information:
l
NIH’s Office of Laboratory Animal Welfare website
l
NIH's Vertebrate Animals Section Worksheet
l
NIH Grants Policy Statement, Section 4.1.1: Animal Welfare Requirement (an applicable Animal
Welfare Assurance will be required if the grantee institution does not have one)
11. Select Agent Research
Who must complete the “Select Agent Research” attachment:
Include a “Select Agent Research” attachment if the proposed training activities will involve the use
of select agents at any time during the proposed project period, either at the applicant
organization or at any performance site.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
For more information:
Select agents are hazardous biological agents and toxins that have been identified by HHS or the
U.S. Department of Agriculture (USDA) as having the potential to pose a severe threat to public
health and safety, to animal and plant health, or to animal and plant products. The Centers of
Disease Control and Prevention (CDC) and the Animal APHIS Select Agent Programs jointly
maintain a list of these agents. See the Federal Select Agent Program website.
See also the NIH Grants Policy Statement, Section 4.1.24.1: Public Health Security and Bioterrorism
Preparedness and Response Act (Select Agents).
Content:
If participating faculty proposed in the training program are conducting or plan to conduct
research involving select agents in which trainees may participate, follow the instructions below.
Excluded select agents: If the activities proposed in the application involve only the use of a
strain(s) of select agents which has been excluded from the list of select agents and toxins as per
M - 152

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
42 CFR 73, the select agent requirements do not apply. Use this “Select Agent Research”
attachment to identify the strain(s) of the select agent that will be used and note that it has been
excluded from this list. The CDC maintains a list of exclusions, which is available on the Select
Agents and Toxins Exclusions website.
Applying for a select agent to be excluded: If the strain(s) is not currently excluded from the list
of select agents and toxins but you have applied or intend to apply to HHS for an exclusion from
the list, use this section to indicate the status of your request or your intent to apply for an
exclusion and provide a brief justification for the exclusion.
All applicants proposing to use select agents: Address the following three points for each site
at which select agent research will take place. Although no specific page limitation applies to this
section, be succinct.
1. Identify the select agent(s) to be used in the proposed research.
2. Provide the registration status of all entities* where select agent(s) will be used.
l
If the performance site(s) is a foreign institution, provide the name(s) of the
country or countries where select agent research will be performed.
l
*An “entity” is defined in 42 CFR 73.1 as “any government agency (federal, state,
or local), academic institution, corporation, company, partnership, society,
association, firm, sole proprietorship, or other legal entity.”
3. Provide a description of all facilities where the select agent(s) will be used.
l
Describe the procedures that will be used to monitor possession, use and transfer
of select agent(s).
l
Describe plans for appropriate biosafety, biocontainment, and security of the
select agent(s).
l
Describe the biocontainment resources available at all performance sites.
12. Consortium / Contractual Arrangements
Who must complete the “Consortium/Contractual Arrangements” attachment:
Include the “Consortium/Contractual Arrangement” attachment if you have consortiums/contracts
in your budget.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page. Use of hyperlinks and
URLs is not allowed in this section unless specified by the funding opportunity announcement.
Content:
Explain the programmatic, fiscal, and administrative arrangements to be made between the
applicant organization and the consortium organization(s). If consortium/contractual activities
represent a significant portion of the overall project, explain why the applicant organization, rather
than the ultimate performer of the activities, should be the grantee.
Note: The signature of the authorized organization representative on the M.200 - SF 424 (R&R)
Form, Authorized Representative signifies that the applicant and all proposed consortium
participants understand and agree to the following statement:
M - 153

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
The appropriate programmatic and administrative personnel of each organization involved in
this grant application are aware of the agency’s consortium agreement policy and are prepared
to establish the necessary inter-organizational agreement(s) consistent with that policy.
For more information:
Refer to the NIH Grants Policy Statement, Section 15: Consortium Agreements for more
information.
13. Other Plan(s)
For NIH Training Grant Applicants, the Data Management and Sharing (DMS) Plan is not
required.
For more information on the DMS Policy see the NIH Data Management and Sharing Policy on
the NIH Scientific Data Sharing website or the NIH Grants Policy Statement, Section 8.2.3.1: Data
Sharing Policy. See also Frequently Asked Questions for additional information on the DMS Policy
on these and other topics.
Additional Instructions for Multi-project:
Overall Component: Include a single consolidated “Data Management and Sharing
Plan” in the Overall Component.
Other Components: Do not include a “Data Management and Sharing Plan” within
other components. Any component-specific information should be described within
the overall “Data Management and Sharing Plan” attachment in the Overall
Component.
Appendix
14. Appendix
Refer to the FOA to determine whether there are any special appendix instructions for your
application. See the updated NIH Guide Notice on the Appendix Policy.
Additional Instructions for Multi-project:
Overall and Other Components: The "Appendix" attachment is optional.
Format:
A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 appendix
attachments are needed, combine the remaining information into attachment #10. Use of
hyperlinks and URLs is not allowed unless specified by the funding opportunity announcement.
As a reminder, tables other than the required Data Tables 1-8 must be incorporated into the
Program Plan (and will count toward the Program Plan’s page limits), and must not be included in
the Appendix. Follow the page limits for Institutional Training Grants specified in the NIHTable of
Page Limits, unless otherwise specified in the FOA.
Use filenames for attachments that are descriptive of the content.
M - 154

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.420 - PHS 398 Research Training Program Plan Form
A summary sheet listing all of the items included in the Appendix is encouraged but not required.
When including a summary sheet, it should be included in the first appendix attachment.
Content:
The only allowable appendix materials are:
l
Blank data collection forms, blank survey forms, and blank questionnaire forms - or
screenshots thereof
l
Simple lists of interview questions
Note: In your blank forms and lists, do not include items such as: data, data
compilations, lists of variables or acronyms, data analyses, publications, manuals,
instructions, descriptions or drawings/figures/diagrams of data collection methods or
machines/devices.
l
Blank informed consent/assent forms
l
Other items only if they are specified in the FOA as allowable appendix materials
No other items are allowed in the Appendix. Simply relocating disallowed materials to other parts
of the application will result in a noncompliant application
Some FOAs may have different instructions for the Appendix. Always follow the instructions in
your FOA if they conflict with these instructions
Note: Applications will be withdrawn and not reviewed if they do not follow the appendix
requirements in these instructions or in your FOA.
Information that expands upon or complements information provided in any section of the
application - even if it is not required for the review - is not allowed in the Appendix unless it is
listed in the allowed appendix materials above or in your FOA. For example, do not include
material transfer agreements (MTA) in the Appendix unless otherwise specified in the FOA.
For more information:
l
The NIH Guide Notice on Reminder: NIH Applications Must Be Complete and Compliant
With NIH Policy and Application Instructions At Time of Submission.
l
Failure of reviewers to address non-required appendix materials in their reviews is not an
acceptable basis for an appeal of initial peer review. For more information, see the NIH
Grants Policy Statement, Section 2.4.2: Appeals of Initial Scientific Review.
l
Appendix Policy Frequently Asked Questions
M - 155

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
M.500 - PHS Human Subjects and
Clinical Trials Information
The PHS Human Subjects and Clinical Trials Information
form is used to collect information on human subjects
research, clinical research, and/or clinical trials, including
study population characteristics, protection and
monitoring plans, and a protocol synopsis.
This form accommodates the full spectrum of all types of
clinical trials, including, but not limited to, behavioral,
exploratory/development, mechanistic, pilot/feasibility,
early phase, efficacy, effectiveness, group-randomized,
and others.
Read all the instructions in the Funding Opportunity
Announcement (FOA) before completing this form to
ensure your application meets all IC-specific criteria.
"Section II. Award Information" of the FOA will indicate
whether clinical trials are or are not allowed and whether
clinical trial research experience is or is not allowed. The
designation of your FOA will determine how to use these instructions, and subsequently, how
to fill out this form.
The PHS Human Subjects and Clinical Trials Information form, together with the rest of your
application, should include sufficient information for the evaluation of the project, independent
of any other documents (e.g., previous application). Be specific, describe each study clearly, and
avoid redundancies. Be especially careful to avoid redundancies with your research strategy.
View larger image
Quick Links
PHS Human Subjects and Clinical Trials Information
Use of Human Specimens and/or Data
If No to Human Subjects
If Yes to Human Subjects
Other Requested Information
Study Record(s)
Delayed Onset Study(ies)
Study Record: PHS Human Subjects and Clinical Trials Information
Section 1 - Basic Information
M - 156

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
1.1 Study Title (each study title must be unique)
1.2 Is this Study Exempt from Federal Regulations?
1.3 Exemption Number
1.4 Clinical Trial Questionnaire
1.5 Provide the ClinicalTrials.gov Identifier (e.g. NCT87654321) for this trial, if applicable.
Section 2 - Study Population Characteristics
2.1 Conditions or Focus of Study
2.2 Eligibility Criteria
2.3 Age Limits
2.3.a Inclusion of Individuals Across the Lifespan
2.4 Inclusion of Women and Minorities
2.5 Recruitment and Retention Plan
2.6 Recruitment Status
2.7 Study Timeline
2.8 Enrollment of First Participant
2.9 Inclusion Enrollment Report(s)
Section 3 - Protection and Monitoring Plans
3.1 Protection of Human Subjects
3.2 Is this a multi-site study that will use the same protocol to conduct non-exempt human
subjects research at more than one domestic site?
3.3 Data and Safety Monitoring Plan
3.4 Will a Data and Safety Monitoring Board be appointed for this study?
3.5 Overall Structure of the Study Team
Section 4 - Protocol Synopsis
4.1 Study Design
4.2 Outcome Measures
4.3 Statistical Design and Power
4.4 Subject Participation Duration
4.5 Will the study use an FDA-regulated intervention?
4.6 Is this an applicable clinical trial under FDAAA?
4.7 Dissemination Plan
Section 5 - Other Clinical Trial-related Attachments
5.1 Other Clinical Trial-related Attachments
M - 157

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Complete the PHS Human Subjects and Clinical Trials Information form after you have completed the
M.220 - R&R Other Project Information Form.
This form accommodates the full spectrum of all types of clinical trials, including, but not limited to,
exploratory/development, mechanistic, pilot/feasibility, early phase, efficacy, effectiveness, group-
randomized, and others.
Who should use the PHS Human Subjects and Clinical Trials Information form:
The designation of your FOA will determine how to use these instructions, and subsequently, how to fill
out this form.
All applicants must use the PHS Human Subjects and Clinical Trials Information form regardless of your
answer to the question “Are human subjects involved?” on the M.220 - R&R Other Project Information
Form.
Note for studies involving only the secondary use of identifiable biospecimens or data: For
studies where the only involvement of human subjects is the use of identifiable biospecimens or data
originally collected for another purpose, complete the PHS Human Subjects and Clinical Trials
Information form with information specific to the current study and not the original collection unless
the information associated with the original collection is pertinent to the proposed study. If information
about the original collection is necessary, provide context and clearly distinguish between the current
study and historical information.
Using the PHS Human Subjects and Clinical Trials Information form:
Everyone must complete the "Use of Human Specimens and/or Data" section of the PHS Human
Subjects and Clinical Trials Information form. However, your answer to the “Are human subjects
involved?” question will determine which other sections of the PHS Human Subjects and Clinical Trials
Information form you must complete. Once you have completed the "Use of Human Specimens and/or
Data" section, follow instructions on the form that are specific to your answer to the "Are human
subjects involved?" question on the M.220 - R&R Other Project Information Form:
l
if you answered "Yes" to the question "Are human subjects involved?" on the M.220 - R&R
Other Project Information Form, see the "If Yes to Human Subjects" section for instructions.
l
if you answered "No" to the question "Are human subjects involved?" on the M.220 - R&R
Other Project Information Form, see the "If No to Human Subjects" section for instructions.
The PHS Human Subjects and Clinical Trials Information form allows you to add Study Record(s) and/or
Delayed Onset Study(ies), as applicable.
Within each Study Record, you will add detailed information at the study level. Do not duplicate studies
within your application. Each study within the application should be unique and should have a unique
study title. Each Study Record is divided into numbered sections:
l
Section 1 - Basic Information
l
Section 2 – Study Population Characteristics (includes Inclusion Enrollment Report)
l
Section 3 – Protection and Monitoring Plans
l
Section 4 – Protocol Synopsis
l
Section 5 – Other Clinical Trial-related Attachments
M - 158

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Note: The PHS Human Subjects and Clinical Trials Information form will capture detailed information at
the study level. Although you are encouraged to refer to information in the PHS Human Subjects and
Clinical Trials Information form in your discussion of the Research Strategy, do not duplicate
information between the Research Strategy attachment and the PHS Human Subjects and Clinical Trials
Information form.
For more information on what a “study” is for the purposes of the PHS Human Subjects and Clinical
Trials Information form, see the relevant FAQ on the Applying Electronically FAQ page.
The PHS Human Subjects and Clinical Trials Information form is dynamic and may eliminate sections
that are not relevant to your application. The dynamic form behavior may not be enabled on all
submission methods.
Note: Some fields in this form match fields within ClinicalTrials.gov and are identified as such within
these instructions. Additional information about the fields can be found on the ClinicalTrials.gov
Protocol Registration Data Element Definitions website.
Additional Instructions for Multi-project:
For multi-project applications with studies that are self-contained within a
single component:
Overall Component: Do not complete a Study Record.
Other Component: Complete a separate Study Record for each human subjects
study that is self-contained within a single component.
For multi-project applications with studies that span components:
Overall Component: Complete one Study Record for each study if it spans multiple
components. This Study Record must include sufficient information for all
components that are involved in the particular study. This might occur when an
application includes a data coordinating center or recruitment core, or when
participant assessments for one study are conducted across multiple components
(e.g., the study includes an imaging core and clinical site).
Applicants must follow all policies and requirements related to formatting, proprietary
information, human subjects, and clinical trials. See the following pages for more information:
l
Format Attachments
l
Rules for Text Fields
l
NIH Grants Policy Statement, Section 2.3.11.2: Confidentiality of Information
l
NIH Grants Policy Statement, Section 2.3.11.2.2: The Freedom of Information Act
l
NIH's Human Subjects Research website
l
NIH's Clinical Trials website
l
Policy on Good Clinical Practice Training for NIHAwardees Involved in NIH-funded Clinical
Trials
Note: There are no page limits for any attachments in the PHS Human Subjects and Clinical Trials
Information form.
M - 159

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
PHS Human Subjects and Clinical Trials
Information
Applicants must complete the human subjects questions on the M.220 - R&R Other Project Information
Form prior to completing this form.
Use of Human Specimens and/or Data
Regardless of your answer to the question “Are Human Subjects Involved?” on the M.220 -
R&R Other Project Information Form, answer the following question(s) about the use of
human specimens and/or human data.
Does any of the proposed research in the application involve human specimens and/or data?
Select “Yes” or “No” to indicate whether the proposed research involves human specimens and/or
data.
Note: Applications involving the use of human specimens or data may not be considered to be
research involving human subjects, depending on the details of the materials to be used.
Provide an explanation for any use of human specimens and / or data not considered to be
human subjects research.
If you answered “No” to the “Does any of the proposed research in the application involve human
specimens and/or data?” question, you do not need to attach an explanation here.
If you answered “Yes” to the “Does any of the proposed research in the application involve human
specimens and/or data?” question, you must provide an explanation for any use of human
specimens and/or data not considered to be human subjects research. To help determine whether
your research is classified as human subjects research, refer to the Research Involving Private
Information or Biological Specimens flowchart. Do not describe use of human specimens and / or
data considered to be human subjects research here. For any human specimens and/or data that is
considered human subjects research, you will add a Study Record. Do not duplicate the
information in your explanation in any of your Study Records.
Attach the explanation as a PDF file. See NIH’s Format Attachments page.
This explanation should include:
l
information on who is providing the data/biological specimens and their role in the
proposed research;
l
a description of the identifiers that will be associated with the human specimens and data;
l
a list of who has access to subjects’ identities; and
l
information about the manner in which the privacy of research participants and
confidentiality of data will be protected.
M - 160

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Please complete the human subjects section of the Research & Related Other
Project Information form prior to completing this form.
Are Human Subjects Involved? Yes/No
This field is pre-populated from the M.220 - R&R Other Project Information Form. If the value in
this field appears to be incorrect, you may correct it by adjusting it on the M.220 - R&R Other
Project Information Form.
Is the Project Exempt from Federal regulations? Yes/No
This field is pre-populated from the M.220 - R&R Other Project Information Form. If the value in
this field appears to be incorrect, you may correct it by adjusting it on the M.220 - R&R Other
Project Information Form.
Exemption number: 1, 2, 3, 4, 5, 6, 7, 8
This field is pre-populated from the M.220 - R&R Other Project Information Form. If the value in
this field appears to be incorrect, you may correct it by adjusting it on the M.220 – R&R Other
Project Information Form.
Note: If you change your answer to the “Are Human Subjects Involved” question on the M.220 - R&R
Other Project Information Form after you have started entering information into the PHS Human
Subjects and Clinical Trials Information form, your data in the PHS Human Subjects and Clinical Trials
Information form may be lost.
If No to Human Subjects
If you answered "No" to the question “Are Human Subjects Involved?” on the M.220 - R&R
Other Project Information Form, skip the rest of the PHS Human Subjects Clinical Trials
Information form unless otherwise directed by your FOA.
If Yes to Human Subjects
If you answered “Yes” to the question “Are Human Subjects Involved?” on the M.220 -
R&R Other Project Information Form, add a Study Record for each proposed study
involving human subjects by selecting “Add New Study” or “Add New Delayed Onset
Study,” as appropriate.
Other Requested Information
Who may provide Other Requested Information:
Follow the instructions below and any instructions in your FOA to determine whether you are
permitted to include the “Other Requested Information” attachment.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page. Hyperlinks and URLs are
not allowed unless specified in the funding opportunity announcement.
M - 161

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Content:
Content is limited to what is described in your FOA or in these instructions. Do not use the “Other
Requested Information” attachment to include any other information.
Renewal applications: When preparing a renewal (or resubmission of a renewal), you can provide
a list of ongoing studies or ClinicalTrials.gov identifiers (e.g., NCT87654321).
Additional Instructions for Multi-project:
For multi-project applications with studies that span components:
Overall Component: For each study that spans components, describe the
components involved with the study.
Other Components: Each component should include an attachment that indicates
that the details of the study are included in the Overall component within this
attachment.
For more information, see the “Where do I enter my human subjects study
information in my multi-project application” FAQ on the Applying Electronically FAQ
page.
Study Record(s)
Adding Study Record Attachment(s):
Add a study record for each proposed study involving human subjects. Projects involving public
health surveillance activities described in 45 CFR 46.102(l)(2) must complete one or more Study
Records describing those public health surveillance activities as if the exclusion does not apply. If
specific plans for your study involving human subjects can be described in the application but will
not begin immediately (i.e., your study has a delayed start), you must add a Study Record for that
study. If your study anticipates involving human subjects within the period of award but specific
plans cannot be described in the application (i.e., delayed onset), see the instructions for Delayed
Onset Study(ies).
For all submission methods, the Study Record is used to collect human subjects study data. Note:
The steps to add a Study Record attachment(s) may vary with the submission method. For
example, from the ASSIST Human Subjects and Clinical Trials tab, use the ‘Add New Study’ button
to access the data entry screens to enter Study Record information directly into ASSIST. With other
submission methods, you may have to extract a blank copy of the Study Record, complete it
offline, and then attach it to your application.
Note on Grouping Studies into Study Records: While there may be more than one way to split
or group studies into Study Records, you are encouraged to group studies that use the same
human subjects population and same research protocols into a single Study Record, to the extent
that the information you provide is accurate and understandable to NIH staff and reviewers.
If information in any attachment is identical across studies, include the complete information only
in the first Study Record for which the information is relevant. In the subsequent Study Records for
which the identical information is needed, upload an attachment that says, “See information for
attachment X in Study Record entitled [include study title]." No other information is needed in the
attachment. Do not submit attachments that are duplicated from one Study Record to another.
Note that you should not name Study Records by number. Examples of attachments that may be
M - 162

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
identical across studies include, but are not limited to, the 3.1 Protection of Human Subjects and
3.5 Overall Structure of the Study Team attachments.
See the NIH Glossary definitions of Study and Study Record.
The PHS Human Subjects and Clinical Trials Information form accommodates up to 150 separate
Study Records.
Format:
All attachments must be PDF files. If you extract a Study Record, it will already be in a fillable PDF
format. Please use this PDF file and do not alter the format of the Study Record file. Use unique
filenames for each human subject study record. The filename for each attachment within a study
must be unique within the application (i.e., do not use the same filename in multiple Study
Records). Use of hyperlinks and URLs is not allowed unless specified in the funding opportunity
announcement.
Content:
Follow the instructions in the “Study Record: PHS Human Subjects and Clinical Trials Information”
section below.
Delayed Onset Study(ies)
If you anticipate conducting research involving human subjects but cannot describe the study at
the time of application (i.e., your study is a delayed onset human subject study), enter a Delayed
Onset Study Record as instructed below.
Generally, for any study that you include as a delayed onset study in this section, you will provide a
study title, indicate whether the study is anticipated to include a clinical trial, and include a
justification attachment. Since by definition, information for a delayed onset study is not available
at the time of application, you will not be given the option to complete a full Study Record for a
delayed onset study. For delayed onset studies, the Delayed Onset Study Record is sufficient.
Notes on delayed onset studies:
l
Delayed onset does NOT apply to a study that can be described but will not start
immediately (i.e., delayed start). Refer to the NIH Glossary definition of Delayed Onset
Study and Delayed Start.
l
If you anticipate multiple delayed onset studies, you can include them together in a single
Delayed Onset Study Record.
Study Title
This field is required.
The Study Title can have a maximum of 600 characters.
Enter a brief, unique title that describes the study the participants will be involved in. Each study
within your application must have a unique Study Title. The first 150 characters will display in the
application image bookmarks.
Note on multiple delayed onset studies: If you are including multiple delayed onset studies in
one delayed onset study entry, you may enter “Multiple Delayed Onset Studies” as the title of this
record.
M - 163

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Anticipated Clinical Trial?
This field is required.
Check this box if you anticipate that this study will be a clinical trial. For help determining whether
your study meets the definition of clinical trial, see the Clinical Trial Questionnaire below.
Read your FOA carefully to determine whether clinical trials are allowed in your application.
Note on multiple delayed onset studies: If you are including multiple delayed onset studies in
one delayed onset study entry, and you anticipate that any of these studies will be a clinical trial,
check the “Anticipated Clinical Trial?” checkbox.
Justification Attachment
This attachment is required.
Attach the justification as a PDF file. See NIH’s Format Attachments page. Use of hyperlinks and
URLs is not allowed unless specified in the funding opportunity announcement.
l
All delayed onset studies must provide a justification explaining why human subjects
study information is not available at the time of application.
l
If NIH’s Policy on the Dissemination of NIH-Funded Clinical Trial Information will apply to
your study, this justification must also include the dissemination plan.
Note on multiple delayed onset studies: If you are including more than one delayed onset
study in any given delayed onset study entry, address all the included studies in a single
justification attachment.
Study Record: PHS Human Subjects and Clinical
Trials Information
Section 1 - Basic Information
Who must complete “Section 1 – Basic Information:”
“Section 1 – Basic Information” is required for all studies involving human subjects.
1.1 Study Title (each study title must be unique)
The “Study Title” field is required.
The Study Title can have a maximum of 600 characters.
Enter a brief title that describes the study the participants will be involved in. If there is more than
one study (i.e., you are including more than one Study Record and/or delayed onset study in your
application), each one must have a unique study title. The first 150 characters will display in the
bookmarks of the application image.
Note: When registering a clinical trial in ClinicalTrials.gov, all study titles across your organization
must be unique.
Note: This field matches a ClinicalTrials.gov field (Official Title).
M - 164

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
1.2 Is this Study Exempt from Federal Regulations?
An answer to the “Is this Study Exempt from Federal Regulations?” question is required.
Indicate whether the study is exempt from Federal regulations for the Protection of Human
Subjects.
For more information, see the NIH's Definition of Human Subjects Research website.
1.3 Exemption Number
The “Exemption Number” field is required if you selected “Yes” to the “Is this Study Exempt from
Federal Regulations?” question.
Select the appropriate exemption number(s) for this particular study. Multiple selections are
permitted. Regardless of whether these exemptions may apply to you in the future, you must fill
out your application following the instructions below.
For more information:
The categories of research that qualify for exemption are defined in the Common Rule for the
Protection of Human Subjects. These regulations can be found at 45 CFR 46.
Need help determining the appropriate exemption number?
l
Refer to NIH's Human Subjects FAQs.
l
See the NIH's Human Subjects Frequently Asked Questions section on Exemptions.
The Office for Human Research Protections (OHRP) guidance states that appropriate use of
exemptions described in 45 CFR 46 should be determined by an authority independent from the
investigators (for more information, see OHRP's Frequently Asked Questions). Institutions often
designate their Institutional Review Board (IRB) to make this determination. Because NIH does not
require IRB approval at the time of application, the exemptions designated often represent the
opinion of the PD/PI, and the justification provided for the exemption by the PD/PI is evaluated
during peer review. See NIH Grants Policy Statement Section 4.1.15 for more information.
1.4 Clinical Trial Questionnaire
The Clinical Trial Questionnaire is required.
Note for basic and mechanistic studies involving human participants: The NIH definition of a
clinical trial encompasses a broad range of studies, including studies using human participants
that aim to understand fundamental aspects of phenomena, the pathophysiology of a disease, or
the mechanism of action of an intervention. This includes many mechanistic studies and studies
submitted to Basic Experimental Studies with Humans FOAs.
Answer “Yes” or “No” to the following questions to determine whether this study involves a clinical
trial. Answer the following questions based only on the study you are describing in this Study
Record.
Note: The answer to question “1.4.a Does the study involve human participants?” will be pre-
populated with “Yes” for all study records. You will not be able to change this answer.
M - 165

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
1.4.a. Does the study involve human participants? Yes/No
1.4.b. Are the participants prospectively assigned to an intervention? Yes/No
1.4.c. Is the study designed to evaluate the effect of the intervention on the participants?
Yes/No
1.4.d. Is the effect that will be evaluated a health-related biomedical or behavioral out-
come? Yes/No
If you answered “Yes” to all the questions in the Clinical Trial Questionnaire, this study meets the
definition of a clinical trial.
Refer to the table below for information about what sections of this form are required, based on
your answers to Question 1.4 "Clinical Trial Questionnaire."
Form Section
If you answered "yes"
to all the questions in
the Clinical Trial
Questionnaire
If you answered "no"
to any of the questions
in the Clinical Trial
Questionnaire
Section 2 - Study Population
Characteristics
Required
Required
Section 3 - Protection and
Monitoring Plans
Required
Required
Section 4 - Protocol Synopsis
Required
Do not complete
Section 5 - Other Clinical Trial-
related Attachments
Required if specified in the
FOA
Do not complete
For more information:
l
NIH Glossary’s definition of an NIH-defined clinical trial
l
NIH's Definition of a Clinical Trial page
l
NIH Definition of Clinical Trials Case Studies page
l
FAQs on the NIH Clinical Trial Definition
l
NIH’s decision tool will help determine whether your human subjects research study is an
NIH-defined clinical trial
l
Your study may also be subject to additional regulations. Read NIH’s Requirements for
Registering & Reporting NIH-funded Clinical Trials in ClinicalTrials.gov.
M - 166

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
1.5. Provide the ClinicalTrials.gov Identifier (e.g., NCT87654321) for this trial, if
applicable
If a clinical trial has already been entered into ClinicalTrials.gov, enter the ClinicalTrials.gov
identifier (e.g., NCT87654321) for this trial. Enter the identifier only if you are proposing to work on
that specific clinical trial. If you are only getting samples and/or data from a clinical trial that has
already been entered into ClinicalTrials.gov, do NOT enter the identifier.
If you are building on an existing study (e.g., ancillary study), enter the ClinicalTrials.gov identifier
only for the ancillary study (if registered separately), not the parent study.
Note: The number you enter in this field should match the ClinicalTrials.gov identifier assigned by
ClinicalTrials.gov.
Section 2 - Study Population Characteristics
Who must complete “Section 2 - Study Population Characteristics:”
All of “Section 2 – Study Population Characteristics” is required (see exceptions for Question 2.7 Study
Timeline and for Question 2.8 Enrollment of First Subject) for all human subjects studies unless the
following applies to you:
l
If you selected only Exemption 4 and no other exemptions on the "1.3 Exemption Number"
question, then “Section 2 – Study Population Characteristics” is not required.
2.1 Conditions or Focus of Study
At least 1 entry is required, and up to 20 entries are allowed (enter each entry on its own line). Each
entry is limited to 255 characters.
Identify the name(s) of the disease(s) or condition(s) you are studying, or the focus of the study. If
available, use appropriate descriptors from NLM's Medical Subject Headings (MeSH) so the
application can be categorized. Include an entry for each condition.
Note: This field matches a ClinicalTrials.gov field (Primary Disease or Condition Being Studied in
the Trial, or the Focus of the Study).
2.2 Eligibility Criteria
List the study’s inclusion and exclusion criteria. To provide a bulleted list, use a dash (or other
character) followed by a space (“- “) at the start of each bullet. Be sure to check the formatting in
the assembled application image. Further explanation or justification should be included in the
Recruitment and Retention plan.
Your text entry is limited to 15,000 characters (but typically needs only 500 characters).
Note: This field matches a ClinicalTrials.gov field (Eligibility Criteria).
For more information about formatting text entry fields, see NIH's Rules for Text Fields page and
the ClinicalTrials.gov's Protocol Registration and Results System User's Guide.
M - 167

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
2.3 Age Limits
Minimum Age
Enter the numerical value for the minimum age a potential participant can be to be eligible for the
study. Provide the relevant units of time (i.e., years, months, weeks, days, hours, or minutes). If
there is no lower limit or no lower limit is known, enter “N/A (No Limit)” and do not enter a unit of
time.
Maximum Age
Enter the numerical value for the maximum age a potential participant can be to be eligible for the
study. Provide the relevant units of time (i.e., years, months, weeks, days, hours, or minutes). If
there is no upper limit or no upper limit is known, enter “N/A (No Limit)” and do not enter a unit of
time.
Note: This field matches a ClinicalTrials.gov field (Age Limits).
2.3.a Inclusion of Individuals Across the Lifespan
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Discuss each of the points listed below. Also include any additional information requested in the
FOA.
You will also have to complete an Inclusion Enrollment Report (IER). Note that you may need to
include multiple IERs for each study. Refer to the instructions for the IER below for more
information.
Inclusion of Individuals Across the Lifespan
For the purposes of the Inclusion of Individuals Across the Lifespan, exclusion of any specific age
or age range group (e.g., children or older adults) should be justified in this section. In addition,
address the following points:
l
Individuals of all ages are expected to be included in all NIH-defined clinical research
unless there are scientific or ethical reasons not to include them. Discuss whether
individuals will be excluded based on age and provide a rationale for the minimum and
maximum age of study participants, if applicable. Additionally, if individuals will be
excluded based on age, provide a scientific or ethical rationale for their exclusion. See the
NIH Policy and Guidelines on the Inclusion of Individuals Across the Lifespan as
Participants in Research Involving Human Subjects for additional information about
circumstances that may justify the exclusion of individuals based on age.
l
Include a description of the expertise of the investigative team for working with
individuals of the ages included, the appropriateness of the available facilities to
accommodate individuals in the included age range, and how the age distribution of
participants will contribute to a meaningful analysis relative to the purpose of the study.
When children are involved in research, the policies under HHS’ 45 CFR 46, Subpart D - Additional
Protections for Children Involved as Subjects in Research apply and must be addressed in the
Protection of Human Subjects attachment.
M - 168

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Existing Datasets or Resources. If you will use an existing dataset, resource, or samples that may
have been collected as part of a different study, you must address inclusion, following the
instructions above. Generally, you must provide details about the sex/gender, race, and ethnicity
of the existing dataset/resource and justify the details as appropriate to the scientific goals of the
proposed study.
For more information about what is considered an existing dataset or resource for inclusion policy,
see the NIH FAQs on Monitoring Inclusion When Working with Existing Datasets and/or
Resources.
For more information, see:
l
NIH Policy Implementation Page on Inclusion Across the Lifespan
l
Inclusion Across the Lifespan: Guidance for Applying the Policy infographic
l
NIH FAQs on Inclusion Across the Lifespan
l
HHS’ 45 CFR 46 Subpart D – Additional Protections for Children
l
NIH Grants Policy Statement, Section 4.1.15.7: Inclusion of Individuals Across the Lifespan
as Participants in Research Involving Human Subjects
2.4 Inclusion of Women and Minorities
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Discuss each of the points listed below and include any additional information requested in the
FOA.
You will also have to complete an Inclusion Enrollment Report (IER). Note that you may need to
include multiple IERs for each study. Refer to the instructions for the IER below for more
information.
Inclusion of Women and Minorities
Address the following points:
l
Describe the planned distribution of subjects by sex/gender, race, and ethnicity.
l
Describe the rationale for selection of sex/gender, racial, and ethnic group members in
terms of the scientific objectives and proposed study design. The description may include,
but is not limited to, information on the population characteristics of the disease or
condition under study.
l
Describe proposed outreach programs for recruiting sex/gender, racial, and ethnic group
members.
l
Inclusion and Excluded Groups: Provide a reason for limiting inclusion of any group by
sex/gender, race, and/or ethnicity. In general, the cost of recruiting certain groups and/or
geographic location alone are not acceptable reasons for exclusion of particular groups.
See the Inclusion of Women and Minorities as Participants in Research Involving Human
Subjects - Policy Implementation Page for more information.
M - 169
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Existing Datasets or Resources. If you will use an existing dataset, resource, or samples that may
have been collected as part of a different study, you must address inclusion, following the
instructions above. Generally, you must provide details about the sex/gender, race, and ethnicity
of the existing dataset/resource and justify the details as appropriate to the scientific goals of the
proposed study.
For more information about what is considered an existing dataset or resource for inclusion policy,
see the NIH FAQs on Monitoring Inclusion When Working with Existing Datasets and/or
Resources.
NIH-Defined Phase III Clinical Trials. If the proposed research includes an NIH-Defined Phase III
Clinical Trial, the “Inclusion of Women and Minorities” attachment MUST address plans for how
sex/gender, race, and ethnicity will be taken into consideration in the design and valid analysis of
the trial. See the instructions for “Valid Analysis” and “Plans to test for Differences in Effect among
Sex/gender, Racial, and/or Ethnic Groups” below.
Additional information about valid analysis is available on the NIH Policy and Guidelines on The
Inclusion of Women and Minorities as Subjects in Clinical Research page.
Valid Analysis (for NIH-Defined Phase III Clinical Trials only):
Address the following issues for ensuring valid analyses:
l
Inclusive eligibility criteria – in general, the cost of recruiting certain groups and/or
geographic location alone are not acceptable reasons for exclusion of particular
groups;
l
Allocation of study participants of both sexes/genders and from different racial and/or
ethnic groups to the intervention and control groups by an unbiased process such as
randomization;
l
Unbiased evaluation of the outcome(s) of study participants; and
l
Use of unbiased statistical analyses and proper methods of inference to estimate and
compare the intervention effects by sex/gender, race, and/or ethnicity, particularly if
prior evidence strongly suggests that such differences exist.
Plan to Test for Differences in Effect among Sex/gender, Racial, and/or Ethnic Groups (for NIH-
Defined Phase III Clinical Trials only):
Applicants also should address whether they plan to test for differences in effect among
sex/gender, racial, and/or ethnic groups and why such testing is or is not appropriate.
This plan must include selection and discussion of one of the following analysis plans:
l
Plans to conduct analyses to detect significant differences in intervention effect
among sex/gender, racial, and/or ethnic subgroups when prior studies strongly
support these significant differences among one or more subgroups, or
l
Plans to include and analyze sex/gender, racial, and/or ethnic subgroups when prior
studies strongly support no significant differences in intervention effect between
subgroups. (Representation of sex/gender, racial, and ethnic groups is not required as
subject selection criteria, but inclusion is encouraged.), or
l
Plans to conduct valid analyses of the intervention effect in sex/gender, racial, and/or
ethnic subgroups (without requiring high statistical power for each subgroup) when
the prior studies neither support nor negate significant differences in intervention
effect among subgroups.
M - 170

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
For more information, see:
l
NIH's Policy Implementation Page on the Inclusion of Women and Minorities
l
HHS’ 45 CFR 46 Subpart B – Additional Protections for Pregnant Women, Fetuses, and
Neonates
l
NIH Grants Policy Statement, Section 4.1.15.8: Inclusion of Women and Minorities as
Subjects in Clinical Research and Reporting Sex/Gender, Racial, and Ethnic Participation
2.5 Recruitment and Retention Plan
Who must complete the "Recruitment and Retention Plan" attachment:
The “Recruitment and Retention Plan” attachment is required unless the following applies to you:
l
You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number”
question.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Describe how you will recruit and retain participants in your study. You should address both
planned recruitment activities as well as proposed engagement strategies for retention.
2.6. Recruitment Status
Who must complete the "Recruitment Status" question:
The “Recruitment Status” question is required unless the following applies to you:
l
You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number”
question.
Content:
From the dropdown menu, select the "Recruitment Status" that best describes the proposed study,
based upon the status of the individual sites. If any facility in a multi-site study has an individual
site status of “recruiting,” then choose “recruiting” for this question. Only one selection is allowed.
Choose from the following options:
l
Not yet recruiting
l
Recruiting
l
Enrolling by invitation
l
Active, not recruiting
l
Completed
l
Suspended
l
Terminated (Halted Prematurely)
l
Withdrawn (No Participants Enrolled)
M - 171

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Note: This field matches a ClinicalTrials.gov field (Overall Recruitment Status).
2.7. Study Timeline
Who must complete the "Study Timeline" attachment:
The “Study Timeline” attachment is required if you answered "Yes" to all the questions in the
"Clinical Trial Questionnaire" (i.e., your study is a clinical trial).
The "Study Timeline" attachment is optional if either of the following apply to you:
l
You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number”
question.
l
You answered "No" to any of the questions in the "Clinical Trial Questionnaire" (i.e., your
study is not a clinical trial).
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Provide a description or diagram describing the study timeline. The timeline should be general
(e.g., "one year after notice of award"), and should not include specific dates.
Note: Additional milestones or timelines may be requested as just-in-time information or post-
award.
2.8. Enrollment of First Participant
Who must complete the "Enrollment of First Participant" question:
Do not complete this field if you will answer "Yes" to the question "Using an Existing Dataset or
Resource" in the Inclusion Enrollment Report.
The “Enrollment of First Participant” question is otherwise required unless the following applies to
you:
l
You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number”
question.
Content:
Enter the date (MM/DD/YYYY) of the enrollment of the first participant into the study. From the
dropdown menu, select whether this date is anticipated or actual.
2.9. Inclusion Enrollment Report(s)
Who must complete the Inclusion Enrollment Report(s):
An Inclusion Enrollment Report is required for all human subjects studies unless, on Question 1.3
“Exemption Number,” you selected only Exemption 4 and no other exemptions.
M - 172

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Using the Inclusion Enrollment Report:
Each proposed study, unless it falls under Exemption 4, must contain at least one Inclusion Enrollment
Report (IER). However, more than one IER per study is allowed.
Once you have added an IER for a given study, you may edit, remove, or view it.
Note: You can add a maximum of 20 IERs per Study Record. These can be a combination of planned
and cumulative reports.
Multi-site studies: Generally, if the application includes a study recruiting subjects at more than one
site/location, investigators may create one IER or separate, multiple IERs to enable reporting by study or
by site, depending on the scientific goals of the study and whether monitoring of inclusion enrollment
would benefit from being combined or separated. At a minimum, participants enrolled at non-U.S. sites
must be reported separately from participants enrolled at U.S. sites, even if they are part of the same
study. Please review the FOA to determine whether there are any other specific requirements about
how to complete the IER.
Duplicative Inclusion Reports: It is important that the IER for a given study be associated with only
one application and be provided only once in a given application (e.g., do not submit the same IER on
both the data coordinating center and the research site). If submitting individual application(s) as part
of a network or set of linked applications, please provide the IER with the individual site applications
unless otherwise directed by the FOA.
Renewal applications: When preparing a renewal (or resubmission of a renewal), investigators should
provide a narrative description regarding the cumulative enrollment from the previous funding period
(s) as part of the progress report section of the research strategy attachment in the application. The IER
should NOT be used for this purpose. If a given study will continue with the same enrollment or
additional enrollment, or if new studies are proposed, provide a new IER for each as described in the
instructions below.
Resubmission applications: If IERs were provided in the initial submission application, and if those
studies will be part of the resubmission application, complete the IER and submit again with the
resubmission application, regardless of whether the enrollment has changed or not. Also, provide any
new (additional) IERs.
Revision applications: Provide an IER if new studies are planned as part of the Revision and they meet
the NIH definition for clinical research.
Additional Instructions for Multi-project:
For multi-project applications with studies that are self-contained within a
single component:
Other Component: Include the IER(s) with the component(s) that involves the study
(s), unless otherwise directed by the FOA.
For multi-project applications with studies that span components:
Overall Component: Should the study span more than one component, include the
IER with the Study Record in the Overall Component and insert a comment in the
comment field of the IER to indicate what other components it is associated with.
For more information:
Refer to the Inclusion of Women and Minorities as Participants in Research Involving Human Subjects -
Policy Implementation Page.
M - 173

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
1. Inclusion Enrollment Report Title
The “Inclusion Enrollment Report Title” field is required.
The “Inclusion Enrollment Report title can have a maximum of 600 characters.
Enter a unique title for each IER. The title should indicate specific criteria that uniquely identify
each report. If the Project Title is pre-populated, you may edit it so that each IER title is unique.
2. Using an Existing Dataset or Resource?
The “Using an Existing Dataset or Resource” question is required.
If the study involves analysis of an existing dataset or resource (e.g., biospecimens) only, answer
“Yes” to this question. If the study involves prospective recruitment or new contact with
participants answer “No” to this question. Use separate IERs for studies involving use of existing
datasets or resources only and for studies that involve prospective recruitment or new contact with
study participants.
For additional guidance on what is considered an existing dataset, refer to the NIH FAQs on
Monitoring Inclusion When Working with Existing Datasets and/or Resources.
3. Enrollment Location Type (Domestic/Foreign)
The “Enrollment Location Type” field is required.
Select whether the participants described in the IER are based at a U.S. (Domestic) or at a non-U.S.
(Foreign) site. Participants at U.S. and non-U.S. sites must be reported separately (i.e., on separate
IERs), even if it is for the same study.
For additional guidance on how to complete the IER if you will be working with non-U.S.
populations, refer to these FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
4. Enrollment Country(ies)
The “Enrollment Country(ies)” field is optional.
Indicate the country or countries in which participants will be enrolled. Multiple U.S. sites can be
reported together in one IER. Foreign countries can be reported together in one IER. However, you
must use separate IERs for U.S. and non-U.S. sites. You can add up to 200 countries per IER.
5. Enrollment Location(s)
The “Enrollment Location(s)” field is optional.
Indicate the type of enrollment location (e.g., hospital, university, or research center), not the name
of the enrollment location.
Enrollment locations are typically where the research is conducted, and can be different from the
recruitment site.
6. Comments
Your comments are limited to 500 characters.
Enter information you wish to provide about this IER. This includes, but is not limited to,
addressing information about distinctive subpopulations if relevant to the scientific hypotheses
being studied. If inclusion monitoring is conducted on another study or NIH grant (e.g., data
coordinating center or research site), please indicate here.
M - 174

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Revision applications: If there are no updates to the IER(s) in your original grant application, do
not include an IER in your Revision application. Instead, provide a comment in this field to the
effect that previous IER(s) are still applicable. If you are revising the IER(s) in your original grant
application, provide a comment here to that effect.
Additional Instructions for Multi-project:
For multi-project applications with studies that span components:
Overall Component: Should the study span more than one component, include the
IER with the Study Record in the Overall Component and insert a comment here in
the comment field to indicate what other components it is associated with.
Planned
Who must complete planned enrollment tables:
All studies must enter planned enrollment counts unless your proposed study will use only an
existing dataset or resource. Planned enrollment generally means that individuals will be recruited
into the study and/or that individuals have already been recruited and continue to be part of the
study.
For more information about what is considered an existing dataset or resource for inclusion policy,
see the NIH FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
For more information on racial categories, see the NIH Glossary definition of Racial Categories.
For more information on ethnic categories, see the NIH Glossary definition of Ethnic Categories.
Racial Categories
American Indian/Alaska Native:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both American
Indian/Alaska Native and Not Hispanic or Latino. Enter the expected number of females and males
(in the respective fields) who are both American Indian/Alaska Native and Hispanic or Latino.
Asian:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both Asian and
Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields)
who are both Asian and Hispanic or Latino.
Native Hawaiian or Other Pacific Islander:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both Native
Hawaiian or Other Pacific Islander and Not Hispanic or Latino. Enter the expected number of
females and males (in the respective fields) who are both Native Hawaiian or Other Pacific Islander
and Hispanic or Latino.
Black or African American:
These fields are required.
M - 175

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Enter the expected number of females and males (in the respective fields) who are both Black or
African American and Not Hispanic or Latino. Enter the expected number of females and males (in
the respective fields) who are both Black or African American and Hispanic or Latino.
White:
These fields are required.
Enter the expected number of females and males (in the respective fields) who are both White and
Not Hispanic or Latino. Enter the expected number of females and males (in the respective fields)
who are both White and Hispanic or Latino.
More than One Race:
These fields are required.
Enter the expected number of females and males (in the respective fields) who both identify with
more than one racial category and are Not Hispanic or Latino. Enter the expected number of
females and males (in the respective fields) who both identify with more than one racial category
and are Hispanic or Latino.
Total:
The total fields at the bottom will be automatically calculated and reflect the totals of all racial
categories for females, males, and individuals of unknown/not reported sex/gender who are Not
Hispanic or Latino and of all racial categories for females, males, and individuals of unknown/not
reported sex/gender who are Hispanic or Latino. The “Total” fields in the right column will be
automatically calculated to total all individuals.
Cumulative (Actual)
Who must complete cumulative (actual) enrollment tables:
You must enter cumulative enrollment counts if your proposed study will use an existing dataset
or resource.
For more information about what is considered an existing dataset or resource for inclusion policy,
see the NIH FAQs on Inclusion on the Basis of Sex/Gender and Race/Ethnicity.
For more information on racial categories, see the NIH Glossary definition of Racial Categories.
For more information on ethnic categories, see the NIH Glossary definition of Ethnic Categories.
Racial Categories
American Indian/Alaska Native:
These fields are required.
Enter the number of females and males (in the respective fields) who are both American
Indian/Alaska Native and Not Hispanic or Latino. Enter the number of females and males (in the
respective fields) who are both American Indian/Alaska Native and Hispanic or Latino. Use the
“Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Asian:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Asian and Not
Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who
M - 176

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
are both Asian and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e.,
race and/or ethnicity is unknown).
Native Hawaiian or Other Pacific Islander:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Native Hawaiian or
Other Pacific Islander and Not Hispanic or Latino. Enter the expected number of females and
males (in the respective fields) who are both Native Hawaiian or Other Pacific Islander and
Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is
unknown).
Black or African American:
These fields are required.
Enter the number of females and males (in the respective fields) who are both Black or African
American and Not Hispanic or Latino. Enter the expected number of females and males (in the
respective fields) who are both Black or African American and Hispanic or Latino. Use the
“Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
White:
These fields are required.
Enter the number of females and males (in the respective fields) who are both White and Not
Hispanic or Latino. Enter the expected number of females and males (in the respective fields) who
are both White and Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e.,
race and/or ethnicity is unknown).
More than One Race:
These fields are required.
Enter the number of females and males (in the respective fields) who both identify with more than
one racial category and are Not Hispanic or Latino. Enter the expected number of females and
males (in the respective fields) who both identify with more than one racial category and are
Hispanic or Latino. Use the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is
unknown).
Unknown or Not Reported:
These fields are required.
Enter the number of females, males, and individuals of unknown/not reported sex/gender (in the
respective fields) whose race is unknown/not reported and who are Not Hispanic or Latino. Enter
the number of females, males, and individuals of unknown/not reported sex/gender (in the
respective fields) whose race is unknown/not reported and who are Hispanic or Latino. Enter the
number of females, males, and individuals of unknown/not reported sex/gender (in the respective
fields) who are both of unknown/not reported race and of unknown/not reported ethnicity. Use
the “Unknown/Not Reported” fields as needed (i.e., race and/or ethnicity is unknown).
Total:
The total fields at the bottom will be automatically calculated and reflect the totals of all racial
categories for females, males, and individuals of unknown/not reported sex/gender who are Not
Hispanic or Latino and of all racial categories for females, males, and individuals of unknown/not
reported sex/gender who are Hispanic or Latino. Use the “Unknown/Not Reported” fields as
M - 177

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
needed (i.e., race and/or ethnicity is unknown). The “Total” fields in the right column will be
automatically calculated to total all individuals.
Section 3 – Protection And Monitoring Plans
Who must complete “Section 3 – Protection and Monitoring Plans:”
All of “Section 3 – Protection and Monitoring Plans” is required for all studies involving human subjects,
unless otherwise noted.
3.1 Protection of Human Subjects
The “Protection of Human Subjects” attachment is required.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Do not use the “Protection of Human Subjects” attachment to circumvent the page limits of the
Research Strategy.
For Human Subjects Research Claiming Exemptions: If you are claiming that your human
subjects research falls under any exemptions, justify why the research meets the criteria for the
exemption(s) that you have claimed. This justification should explain how the proposed research
meets the criteria for the exemption claimed. Do not merely repeat the criteria or definitions
themselves.
For Studies that involve Non-Exempt Human Subjects Research: For any proposed non-
exempt study involving human subjects, NIH requires a Protection of Human Subjects attachment
that is commensurate with the risks of the study, its size, and its complexity. Organize your
attachment into four sections, following the headings and specified order below, and discuss each
of the points listed below. Start each section with the appropriate section heading – Risks to
Human Subjects, Adequacy of Protection Against Risks, Potential Benefits of the Proposed
Research to Research Participants and Others, and Importance of the Knowledge to be Gained.
Also include any additional information requested in the FOA.
1. Risks to Human Subjects
a. Human Subjects Involvement, Characteristics, and Design
l
Briefly describe the overall study design.
l
Describe the subject population(s) to be included in the study; the procedures for assignment
to a study group, if relevant; and the anticipated numbers of subjects for each study group.
l
List any collaborating sites where human subjects research will be performed, and describe the
role of those sites and collaborating investigators in performing the proposed research.
b. Study Procedures, Materials, and Potential Risks
l
Describe all planned research procedures (interventions and interactions) involving study
subjects; how research material, including biospecimens, data, and/or records, will be obtained;
and whether any private identifiable information will be collected in the proposed research
project.
M - 178

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
l
For studies that will include the use of previously collected biospecimens, data or records,
describe the source of these materials, whether these can be linked with living individuals, and
who will be able to link the materials.
l
Describe all the potential risks to subjects associated with each study intervention, procedure
or interaction, including physical, psychological, social, cultural, financial, and legal risks; risks
to privacy and/or confidentiality; or other risks. Discuss the risk level and the likely impact to
subjects.
l
Where appropriate, describe alternative treatments and procedures, including their risks and
potential benefits. When alternative treatments or procedures are possible, make the rationale
for the proposed approach clear.
2. Adequacy of Protection Against Risks
a. Informed Consent and Assent
l
Describe the process for obtaining informed consent. Include a description of the
circumstances under which consent will be sought and obtained, who will seek it, the nature of
the information to be provided to prospective subjects, and the method of documenting
consent. When appropriate, describe how potential adult subjects’ capacity to consent will be
determined and the plans for obtaining consent from a legally authorized representative for
adult subjects not able to consent.
o
For research involving children: If the proposed studies will include children, describe
the process for meeting HHS regulatory requirements for parental permission and child
assent (45 CFR 46.408). See the HHS page on Research with Children FAQs and the NIH
page on Requirements for Child Assent and Parent/Guardian Permission.
l
If a waiver of some or all of the elements of informed consent will be sought, provide
justification for the waiver. Do not submit informed consent document(s) with your application
unless you are requested to do so.
b. Protections Against Risk
l
Describe planned strategies for protecting against or minimizing all potential risks identified,
including strategies to manage and protect the privacy of participants and confidentiality of
research data.
l
Where appropriate, discuss plans for ensuring necessary medical or professional intervention in
the event of adverse effects on participants.
l
Describe plans for handling incidental findings, such as those from research imaging, screening
tests, or paternity tests.
c. Populations that are vulnerable to coercion or undue influence and pregnant women, fetuses
and neonates, if relevant to your study
Explain the rationale for the involvement of populations that are vulnerable to coercion or undue
influence, such as children, prisoners, individuals with impaired decision-making capacity, or
economically or educationally disadvantaged persons or others who may be considered vulnerable
populations. 'Prisoners' includes all subjects involuntarily incarcerated (for example, in detention
centers). Additionally, explain the rationale for the involvement of pregnant women, human fetuses and
neonates.
M - 179

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Pregnant Women, Fetuses, and Neonates or Children
If the study involves subjects afforded additional protections under Subparts B and D (pregnant women,
fetuses, and neonates or children), provide a clear description of the risk level and additional
protections necessary to meet the HHS regulatory requirements.
l
HHS’ Subpart B - Additional Protections for Pregnant Women, Fetuses, and Neonates
l
HHS’ Subpart D - Additional Protections for Children
l
OHRP Guidance on Subpart D Special Protections for Children as Research Subjects and the
HHS 407 Review Process
Prisoners
If the study involves vulnerable subjects afforded additional protections under Subpart C (prisoners),
describe how proposed research meets the additional regulatory requirements, protections, and plans
to obtain OHRP certification for the involvement of prisoners in research.
Refer to HHS regulations, and OHRP guidance:
l
HHS’ Subpart C - Additional Protections Pertaining to Prisoners as Subjects
l
OHRP Subpart C Guidance on Involvement of Prisoners in Research
3. Potential Benefits of the Proposed Research to Research Participants and Others
l
Discuss the potential benefits of the research to research participants and others.
l
Discuss why the risks to subjects are reasonable in relation to the anticipated benefits to
research participants and others.
l
Note: Financial compensation of subjects should not be presented as a benefit of participation
in research.
4. Importance of the Knowledge to be Gained
l
Discuss the importance of the knowledge to be gained as a result of the proposed research.
l
Discuss why the risks to subjects are reasonable in relation to the importance of the knowledge
that reasonably may be expected to result.
For more information:
l
Refer to the NIH’s Human Subjects Research website.
3.2 Is this a multi-site study that will use the same protocol to conduct non-
exempt human subjects research at more than one domestic site?
Select "Yes" or "No" to indicate whether this is a multi-site study that will use the same protocol to
conduct non-exempt human subjects research at more than one domestic site.
Select “N/A” only if any of the following apply (do not select “N/A” if none of the following apply):
l
You answered “Yes” to “Question 1.2 Is this Study Exempt from Federal Regulations?
(Yes/No)”
l
You are a training grant applicant.
M - 180

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Applicants who check “Yes” and are subject to the revised Common Rule are expected to use a
single Institutional Review Board (sIRB) to conduct the ethical review required by HHS regulations
for the Protections of Human Subjects Research unless review by a sIRB would be prohibited by
law (including tribal law passed by the official governing body of an American Indian or Alaska
Native tribe).
Applicants who check “Yes” and are subject only to the NIH sIRB policy are expected to use a
single Institutional Review Board (sIRB) to conduct the ethical review required by HHS regulations
for the Protections of Human Subjects Research unless review by a sIRB would be prohibited by a
federal, tribal, or state law, regulation, or policy.
Note: The NIH sIRB policy applies to participating domestic sites. Foreign sites
participating in NIH-funded, multi-site studies are not expected to follow this policy.
For more information:
l
HHS regulations and requirements for the Protections of Human Subjects can be found at
45 CFR 46.
l
See NIH’s Single IRB Policy for Multi-site Research for more information.
l
See the FAQ about answering “No” for this question on the Applying Electronically FAQ
page.
Single IRB Plan Attachment
For NIHApplicants, the single IRBplan is no longer required. See additional information in
the content section below.
For AHRQ applicants, if this is a research project that involves more than one institution and that
will be conducted in the United States, Applicants are expected to use a single Institutional Review
Board (sIRB) to conduct the ethical review required by HHS regulations for the Protections of
Human Subjects Research, and include a single IRB plan as instructed below, unless review by a
sIRB would be prohibited by a federal, tribal, or state law, regulation, or policy.
Note: The sIRB requirement applies to participating sites in the United States. Foreign sites
participating in AHRQ-funded, cooperative research studies are not expected to follow this
requirement.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Although one sIRB plan attachment per application is sufficient, you must include a file for each
study within your application. All filenames within your application must be unique. You may either
attach the same sIRB plan (with different filenames) to different studies or attach a file that refers
to the sIRB plan in another study within your application. For example, you may attach a file that
says “See sIRB plan in the 'My Unique Study Name' study.”
Content:
For NIHapplicants, the single IRB plan is no longer required. Do not provide an attachment.
The applicant must provide a statement naming the sIRB of record in the Just-in-Time submission
prior to award.
M - 181

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
For more information:
l
NIH's Single IRB Policy for Multi-site Research page
l
NIH's FAQs on Single IRB Policy for Multi-site Research
l
NIH's Office of Science Policy's FAQs on NIH Policy on the Use of a Single IRB for Multi-
Site Research Costs
l
NIH's Office of Science Policy's FAQs on Implementation of the sIRB policy
For AHRQ applicants, the single IRB plan should include the following elements:
l
Describe how you will comply with the single IRB review requirement under the Revised
Common Rule at 45 CFR 46.114 (b) (cooperative research). If available, provide the name of the
IRB that you anticipate will serve as the sIRB of record.
l
Indicate that all identified participating sites will agree to rely on the proposed sIRB and that
any sites added after award will rely on the sIRB.
l
Briefly describe how communication between sites and the sIRB will be handled.
l
Indicate that all participating sites will, prior to initiating the study, sign an
authorization/reliance agreement that will clarify the roles and responsibilities of the sIRB and
participating sites.
l
Indicate which institution or entity will maintain records of the authorization/reliance
agreements and of the communication plan.
l
Note: Do not include the authorization/reliance agreement(s) or the communication plan(s)
documents in your application.
l
Note: If you anticipate research involving human subjects but cannot describe the study at the
time of application, include information regarding how the study will comply with the single
Institutional Review Board (sIRB) requirement prior to initiating any multi-site study in the
delayed onset study justification.
For Studies with Legal-, Regulatory-, or Policy-based Claims for Exception as described by
the sIRB Policy: Indicate that review by a sIRB will not be possible for all or some sites (specify
which sites) because local IRB review is required by an existing federal/state/tribal law or policy.
Include a specific citation to the relevant law, policy, or regulation.
For more information:
• AHRQ Guide Notice on Single IRB
• AHRQ Protection of Human Subjects page
3.3 Data and Safety Monitoring Plan
A “Data and Safety Monitoring Plan” attachment is required if you answered “Yes” to all the
questions in the “Clinical Trial Questionnaire.” The “Data and Safety Monitoring Plan” attachment
is optional for all other human subjects research.
For human subjects research that does not involve a clinical trial: Your study, although it is
not a clinical trial, may have significant risks to participants, and it may be appropriate to include a
M - 182

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
data and safety monitoring plan. If you choose to include a data and safety monitoring plan, you
may follow the content criteria listed below, as appropriate.
For AHRQ Applicants, Data and Safety Monitoring (DSM) plans are required in all non-exempt
research applications when support is sought to study the effect of a health-related intervention
on outcomes in human subjects where there is greater than minimal risk.
If you seek AHRQ support to conduct non-exempt research to study the effect of a health-related
intervention on outcomes in human subjects where there is greater than minimal risk, a “Data and
Safety Monitoring Plan” attachment is required.
Refer to AHRQ Data and Safety Monitoring Policy
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
For any proposed clinical trial, NIH requires a data and safety monitoring plan (DSMP) that is
commensurate with the risks of the trial, its size, and its complexity. Provide a description of the
DSMP, including:
l
Indicate how many people and what type of entity will provide the monitoring. Include
such details as whether a single person, multiple people, or a data safety monitoring
board will provide monitoring. Also indicate what type of entity will provide the
monitoring (e.g., PD/PI, Independent Safety Monitor/Designated Medical Monitor,
Independent Monitoring Committee, Safety Monitoring Committee, Data and Safety
Monitoring Board, etc.).
l
The overall framework for safety monitoring and what information will be monitored.
l
The frequency of monitoring, including any plans for interim analysis and stopping rules
(if applicable).
l
The process by which Adverse Events (AEs), including Serious Adverse Events (SAEs) such
as deaths, hospitalizations, and life threatening events and Unanticipated Problems (UPs),
will be managed and reported, as required, to the IRB, the person or group responsible for
monitoring, the awarding IC and the Food and Drug Administration.
l
The individual(s) or group that will be responsible for trial monitoring and advising the
appointing entity. Because the DSMP will depend on potential risks, complexity, and the
nature of the trial, a number of options for monitoring are possible. These include, but are
not limited to, monitoring by a:
o
PD/PI: While the PD/PI must ensure that the trial is conducted according to the
approved protocol, in some cases (e.g., low risk trials, not blinded), it may be
acceptable for the PD/PI to also be responsible for carrying out the DSMP.
o
Independent safety monitor/designated medical monitor: a physician or other expert
who is independent of the study.
o
Independent Monitoring Committee or Safety Monitoring Committee: a small group
of independent experts.
o
Data and Safety Monitoring Board (DSMB): a formal independent board of experts
including investigators and biostatisticians. NIH requires the establishment of DSMBs
for multi-site clinical trials involving interventions that entail potential risk to the
M - 183

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
participants, and generally, for all Phase III clinical trials, although Phase I and Phase II
clinical trials may also need DSMBs. If a DSMB is used, please describe the general
composition of the Board without naming specific individuals.
For more information:
l
NIH Grants Policy Statement, Section 4.1.15.6: Data and Safety Monitoring
l
NIHData and Safety Monitoring Policies
l
NIH Policies and IC Guidance for Data and Safety Monitoring of Clinical Trials
3.4 Will a Data and Safety Monitoring Board be appointed for this study?
The “Data Safety and Monitoring Board” question is required if you answered “Yes” to all the
questions in the “Clinical Trial Questionnaire.” This question is optional for all other human
subjects research.
Check the appropriate box to indicate whether a Data Safety and Monitoring Board (DSMB) will be
appointed for this study.
3.5 Overall Structure of the Study Team
The “Overall Structure of the Study Team” attachment is optional. Refer to your specific FOA for
specific instructions on the "Overall Structure of the Study Team" attachment.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Provide a brief overview of the organizational/administrative structure and function of the study
team, particularly the administrative sites, data coordinating sites, enrollment/participating sites,
and any separate laboratory or testing centers. The attachment may include information on study
team composition and key roles (e.g., medical monitor, data coordinating center), the governance
of the study, and a description of how study decisions and progress are communicated and
reported.
Note: Do not include study team members’ individual professional experiences (i.e., biosketch
information).
Section 4 – Protocol Synopsis
Who must complete “Section 4 – Protocol Synopsis:"
If you answered "Yes" to all the questions in the "Clinical Trial Questionnaire:" All the questions
in the “Protocol Synopsis” section are required.
If you answered “No” to any question in the “Clinical Trial Questionnaire:” Do not provide
information in this section. Inputting information in this section will result in errors and will prevent your
application from being accepted.
M - 184

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
4.1. Study Design
4.1.a. Detailed Description
Enter a narrative description of the protocol. Studies differ considerably in the methods used to
assign participants and deliver interventions. Describe your plans for assignment of participants
and delivery of interventions. You will also need to show that your methods for sample size and
data analysis are appropriate given those plans. For trials that randomize groups or deliver
interventions to groups, special methods are required; additional information is available at the
Research Methods Resources webpage. The Narrative Study Description is not meant to be a
repeat of the Research Strategy.
The narrative description is limited to 32,000 characters (but typically needs only 5,000 characters),
should be written in layperson’s terms, and may repeat some of the information in the Research
Strategy.
Note: This field matches a ClinicalTrials.gov field (Detailed Description).
For more information about formatting text entry fields, see NIH's Rules for Text Fields page.
4.1.b. Primary Purpose
Enter or select from the dropdown menu a single "Primary Purpose" that best describes the clinical
trial. Choose from the following options:
l
Treatment
l
Prevention
l
Diagnostics
l
Supportive Care
l
Screening
l
Health Services Research
l
Basic Science
l
Device Feasibility
l
Other (If you select “Other,” provide a description in the space provided. Your response is
limited to 255 characters.)
Note: This field matches a ClinicalTrials.gov field (Primary Purpose).
4.1.c. Interventions
Complete the “Interventions” fields for each intervention to be used in your proposed protocol. If
an arm of the study to which subjects will be assigned (as discussed in 4.1.a. Detailed Description)
includes more than one intervention (e.g., drug plus educational intervention), complete this
section for each intervention. You can add up to 20 interventions.
Intervention Type: Enter or select from the dropdown menu the intervention type the clinical trial
will administer during the proposed award. Choose from the following options:
l
Drug (including placebo)
l
Device (including sham)
l
Biological/Vaccine
l
Procedure/Surgery
M - 185

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
l
Radiation
l
Behavioral (e.g., Psychotherapy, Lifestyle Counseling)
l
Genetic (including gene transfer, stem cell, and recombinant DNA)
l
Dietary Supplement (e.g., vitamins, minerals)
l
Combination Product
l
Diagnostic Test
l
Other
Name: Enter the name of the intervention. The name is limited to 200 characters.
Description: Enter a description of the intervention. The description is limited to 1,000 characters.
Note: This field matches a ClinicalTrials.gov field. (Interventions, including Intervention Type and
Intervention Name(s)).
For more information on how to answer this question for behavioral research trials, refer to the
relevant FAQ on the Applying Electronically FAQ page.
4.1.d. Study Phase
Enter or select from the dropdown menu a "Study Phase" that best describes the clinical trial. If
your study involves a device or behavioral intervention, choose “N/A”.
Choose from the following options:
l
Early Phase 1 (or Phase 0)
l
Phase 1
l
Phase 1/2
l
Phase 2
l
Phase 2/3
l
Phase 3
l
Phase 4
l
N/A
Is this an NIH-defined Phase III clinical trial? Yes/No
Select "Yes" or "No" to indicate whether the study includes an NIH-defined Phase III clinical trial.
Device and behavioral intervention studies may select “Yes” here even if the answer above is
“Other”.
For more information on how to answer this question for devices or behavioral interventions,
refer to the relevant FAQ on the Applying Electronically FAQ page.
4.1.e. Intervention Model
Enter or select from the dropdown menu a single "Intervention Model" that best describes the
clinical trial. If you select “Other,” provide a description in the space provided. Choose from the
following options:
l
Single Group
l
Parallel
l
Cross-Over
M - 186

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
l
Factorial
l
Sequential
l
Other (If you select “Other,” provide a description in the space provided. Your response is
limited to 255 characters.)
Note: This field matches a ClinicalTrials.gov field (Interventional Study Model).
For more information: Definitions of intervention models may be found in ClinicalTrials.gov’s
Glossary of Common Site Terms or in the ClinicalTrials.gov’s description of Study Design.
4.1.f. Masking
Select "Yes" or "No" to indicate whether the protocol uses masking. Note that masking is also
referred to as “blinding.”
If you answered “Yes” to the “Masking” question, select one or more types of masking that best
describes the protocol. Choose from the following options:
l
Participant
l
Care Provider
l
Investigator
l
Outcomes Assessor
Note: This field matches a ClinicalTrials.gov field (Masking).
4.1.g. Allocation
Enter or select from the dropdown menu a single "Allocation" that best describes how subjects will
be assigned in your protocol. If allocation is not applicable to your clinical trial, select “N/A” (e.g.,
for a single-arm trial). Choose from the following options:
l
N/A
l
Randomized
l
Non-randomized
Note: This field matches a ClinicalTrials.gov field (Allocation).
4.2. Outcome Measures
Complete the “Outcome Measures” fields for each primary, secondary, and other important
measures to be collected during your proposed clinical trial. You may have more than one primary
outcome measure, and you can add up to 50 outcome measures.
Name: Enter the name of the individual outcome measure. The outcome measure must be unique
within each Study Record.
Type: Enter or select from the dropdown menu the type of the outcome measure. Choose from
the following options:
l
Primary – select this option for the outcome measures specified in your protocol that are
of greatest importance to your study
l
Secondary – select this option for outcome measures specified in your protocol that are of
lesser importance to your study than your primary outcomes
M - 187

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
l
Other – select this option for additional key outcome measures used to evaluate the
intervention.
Time Frame: Indicate when a measure will be collected for analysis (e.g., baseline, post-
treatment).
Brief Description: Describe the metric used to characterize the outcome measure if the metric is
not already included in the outcome measure name. Your description is limited to 999 characters.
NIH-Defined Phase III Clinical Trials: If the proposed research includes an NIH-Defined Phase III
Clinical Trial, then outcomes for required analyses by sex/gender, race, and ethnicity should be
entered.
Additional information about valid analysis is available on the NIH Policy and Guidelines on The
Inclusion of Women and Minorities as Subjects in Clinical Research page.
Note: This field matches a ClinicalTrials.gov field (e.g., Primary Outcome Measure Information,
which includes Title, Description, and Time Frame).
For more information:
l
Refer to the relevant FAQ for question 4.2 Outcome Measures on the Applying
Electronically FAQ page.
4.3. Statistical Design and Power
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Content:
Specify the number of subjects you expect to enroll, the expected effect size, the power, and the
statistical methods you will use with respect to each outcome measure you listed in 4.2 Outcome
Measures.
You will need to show that your methods for sample size and data analysis are appropriate given
your plans for assignment of participants and delivery of interventions. For trials that randomize
groups or deliver interventions to groups, special methods are required; additional information is
available at the Research Methods Resources webpage.
4.4 Subject Participation Duration
Enter the time (e.g., in months) it will take for each individual participant to complete all study
visits. If the participation duration is unknown or not applicable, write “unknown” or “not
applicable.” The subject participation duration is limited to 255 characters.
4.5 Will the study use an FDA-regulated intervention?
Select "Yes" or "No" to indicate whether the study will use an FDA-regulated intervention (see the
definition of “FDA Regulated Intervention” under the Oversight section of the ClinicalTrials.gov
Protocol Registration Data Element Definitions for Interventional and Observational Studies page).
M - 188

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
4.5.a. If yes, describe the availability of Investigational Product (IP) and Investigational New
Drug (IND)/Investigational Device Exemption (IDE) status:
This attachment is required if you answered “Yes” to the “Will the study use an FDA-regulated
intervention?” question.
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
This attachment’s typical length is approximately 3,000 characters.
Content:
Provide a summary describing the availability of study agents and support for the acquisition and
administration of the study agent(s).
Please indicate, if applicable, the IND/IDE status of the study agent, including whether a clinical
investigation is exempt from the IND/IDE requirement. Also indicate whether the investigators
have had any interactions with the FDA (e.g., indicate if the FDA has stated that research may
proceed). If the study agent currently has an IND/IDE number, provide that information.
Do not include the IND/IDE application, manufacturer’s product specifications, study protocol, or
protocol amendments in this attachment.
Additional information such as FDA letters or correspondence with the FDA may be requested in
the FOA.
Note: The awarding component may request consultation with the FDA and the IND/IDE sponsor
about the proposed clinical trial after peer review and prior to award.
4.6 Is this an applicable clinical trial under FDAAA?
Select "Yes" or "No" to indicate whether the study is an applicable clinical trial (ACT) under the
Food and Drug Administration Amendments Act (FDAAA).
For more information:
l
NIH Glossary’s definition of an applicable clinical trial
l
FAQs on the ClinicalTrials.gov & FDAAA
l
ClinicalTrials.gov FAQs
4.7 Dissemination Plan
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
Although one Dissemination Plan per application is sufficient, you must include a file for each
study within your application. All filenames within your application must be unique. You may either
attach the same Dissemination Plan to different studies or attach a file that refers to the
Dissemination Plan in another study within your application. For example, you may attach a file
that says “See Dissemination Plan in the 'My Unique Study Name' study.”
M - 189

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Content:
Explain briefly your plan for the dissemination of NIH-funded clinical trial information and address
how the expectations of the policy will be met. The plan must contain sufficient information to
assure the following:
l
the applicant will ensure that clinical trial(s) under the award are registered and results
information is submitted to ClinicalTrials.gov as outlined in the policy and according to
the specific timelines stated in the policy;
l
informed consent documents for the clinical trial(s) will include a specific statement
relating to posting of clinical trial information at ClinicalTrials.gov; and
l
the recipient institution has an internal policy in place to ensure that clinical trials
registration and results reporting occur in compliance with policy requirements.
Note: Do not include informed consent documents in the Dissemination Plan attachment.
Note: If your human subjects study meets the definition of “Delayed Onset,” include the
Dissemination Plan attachment in the delayed onset study justification.
For more information:
l
See the NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information
l
See the NIH Guide Notice on the Delayed Enforcement and Short-Term Flexibilities for
Some Requirements Affecting Prospective Basic Science Studies Involving Human
Participants
l
See the NIH Grants Policy Statement, Section 4.1.3.1 NIH Policy on Dissemination of NIH-
Funded Clinical Trial Information.
Section 5 – Other Clinical Trial-related Attachments
Who must complete “Section 5 – Other Clinical Trial-related Attachments:"
If you answered “Yes” to all the questions in the “Clinical Trial Questionnaire:” Include an
attachment only if your FOA specifies that an attachment(s) is required or permitted; otherwise, do not
include any Other Clinical Trial-related attachments.
If you answered “No” to any question in the “Clinical Trial Questionnaire:” Do not provide
information in this section. Inputting information in this section will result in errors and will prevent your
application from being accepted.
5.1 Other Clinical Trial-related Attachments
Format:
Attach this information as a PDF file. See NIH’s Format Attachments page.
A maximum of 10 PDF attachments is allowed in the “Other Clinical Trial-related Attachments”
section.
M - 190

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.500 - PHS Human Subjects and Clinical Trials Information
Content:
Provide additional trial-related information only if your FOA specifically requests it. Include only
attachments requested in the FOA, and use requested filenames. If a specific filename is not given
in the FOA, use a meaningful filename since it will become a bookmark in the assembled
application image. Each attachment included in the application must have a unique filename. Do
not use the same file name in multiple study records. If the FOA requires a specific filename, add
unique numbers at the end of the filenames for each study record (e.g. study_filename1, study_
filename2). File name sizes are limited to 50 characters.
M - 191
Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.600 - PHS Assignment Request Form
M.600 - PHS Assignment Request Form
The PHS Assignment Request Form may be used to
communicate specific application assignment and review
preferences to the Division of Receipt and Referral (DRR)
and to Scientific Review Officers (SROs).
This information will not be part of your assembled
application, and it will neither be made available to
program staff nor provided to reviewers. It is used
specifically to convey additional, optional information
about your preference(s) for assignment and review of
your application to DRR and SROs.
View larger image
Completing the PHS Assignment Request Form:
This form is optional. Use it only if you wish to communicate
specific awarding component assignments or review preferences. There is no requirement that all fields
or all sections be completed. You have the flexibility to make a single entry or to provide extensive
information using this form.
Note on Application Assignments: The Division of Receipt and Referral (DRR), Center for Scientific
Review (CSR) is responsible for assigning applications to awarding components such as NIH
Institutes/Centers (ICs) and other PHS agencies for funding consideration. DRR also assigns applications
to NIH Scientific Review Groups (SRGs) and Special Emphasis Panels (SEPs).
Awarding Component Assignment Suggestions (optional)
To facilitate accurate communication of any assignment preferences to NIH referral and review
staff, use the short abbreviation (e.g., NCI for the National Cancer Institute).
All assignment suggestions will be considered; however, not all assignment suggestions can be
honored. Applications are assigned based on relevance of your application to an individual
awarding component mission and scientific interests in addition to administrative requirements
such as IC participation in the funding opportunity announcement used to submit your
application.
Descriptions of the scientific areas covered by all NIH ICs and links to other PHS agency
information can be found on the PHS Assignment Information website.
You do not need to make entries in all three boxes of the “Awarding Component Assignment
Suggestions” section.
Suggested Awarding Component(s):
You may enter up to three preferences for primary assignment in the boxes in the “Suggested
Awarding Component(s)” row. Note: Your application will be assigned based on the most
M - 192

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.600 - PHS Assignment Request Form
appropriate match between it, the terms of the FOA, and the mission of each possible awarding
component, with your preference(s) taken into consideration when possible.
Study Section Assignment Suggestions (optional)
To facilitate accurate communication of any review assignment preferences to NIH referral and
review staff, use the short abbreviation of the SRG/SEP you would prefer. For example, enter
“CAMP” for the NIH Cancer Molecular Pathobiology study section or enter “ZRG1HDMR” for the
NIH Healthcare Delivery and Methodologies SBIR/STTR panel for informatics. Be careful to remove
all hyphens, parentheses, and spaces when you type in the suggestion. Freeform text (such as
"special emphasis panel" or "member conflict SEP") should not be entered.
All suggestions will be considered; however, not all assignment suggestions can be honored.
More information about how to identify CSR and NIH SRGs and SEPs, including their short
abbreviations, can be found on CSR Study Sections and Special Emphasis Panel. A list of all NIH
SRGs and SEPs is also available.
While the majority of NIH research grant and fellowship applications are reviewed by CSR, some
are assigned to individual IC review groups and some are clustered for review in SRGs/SEPs,
depending on existing locus of review agreements within NIH and other PHS agencies. This limits
flexibility for honoring assignment preferences.
You do not need to make an entry in all three boxes of the "Study Section Assignment
Suggestions" section.
Suggested Study Sections:
You may enter up to three preferences for SRGs/SEPs in the boxes in the “Suggested Study
Sections” row. Use one box per individual SRG/SEP preference suggestion. All review preferences
will be considered. Note: Your application will be assigned based on the most appropriate match
between it, the terms of the FOA, and the guidelines for each SRG/SEP, with your preference(s)
taken into consideration when possible.
Note: This information is not applicable if you are submitting an application to an RFA.
Rationale for assignment suggestions (optional)
Enter the rationale (i.e., why you think the assignment is appropriate) for your Awarding
Component and Study Section suggestions.
Your answer can have a maximum of 1000 characters.
List individuals who should not review your application and why (optional)
You may list specific individuals, if any, who should not review your application and why they
should not review your application. Provide sufficient information (e.g., name, organizational
affiliation) so that the SRO can correctly identify the individual. Be prepared to provide additional
information to the SRO if needed. Simply stating “Dr. John Smith is in conflict with my application”
is not helpful.
Your answer can have a maximum of 1000 characters.
M - 193

Multi-Project Instructions for NIHand Other PHSAgencies - Forms Version H Series
M.600 - PHS Assignment Request Form
Identify scientific areas of expertise needed to review your application (optional)
You may list up to five general or specific types of expertise needed for the review of your
application. Limit your answers to areas of expertise – do not enter names of individuals you would
like to review your application.
Each field can have a maximum of 40 characters.
M - 194
Form Screenshots
M.- i
Form Screenshots
Quick Links
SF 424 (R&R) Form
PHS 398 Cover Page Supplement Form
R&R Other Project Information Form
Project/Performance Site Location(s) Form
R&R Senior/Key Person Profile (Expanded) Form
R&R Budget Form
R&R Subaward Budget Attachment(s) Form
PHS 398 Training Budget Form
PHS 398 Training Subaward Budget Attachment(s) Form
PHS Additional Indirect Cost Form
PHS 398 Research Plan Form
PHS 398 Career Development Award Supplemental Form
PHS 398 Research Training Program Plan Form
PHS Human Subjects and Clinical Trials Information
PHS Assignment Request Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- ii
SF 424 (R&R)Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- iii
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series
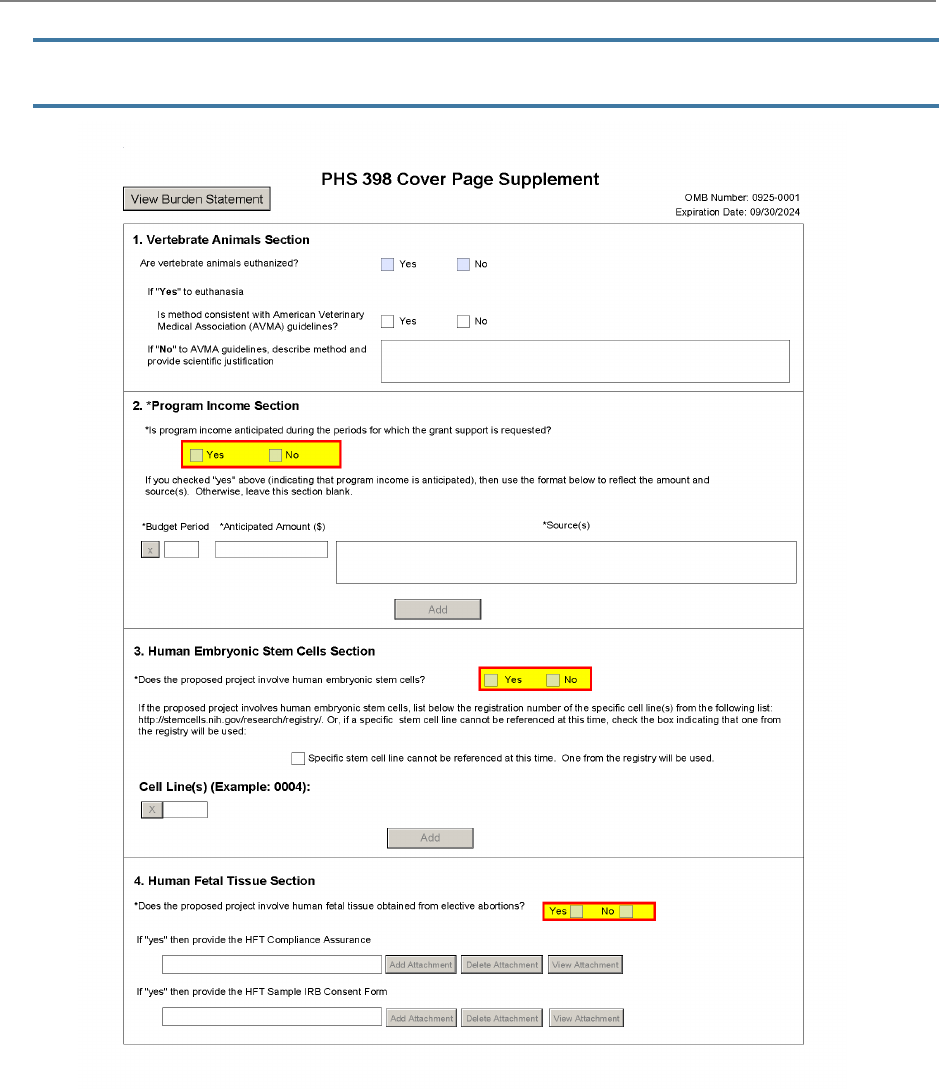
Form Screenshots
M.- iv
PHS 398 Cover Page Supplement Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- v
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series
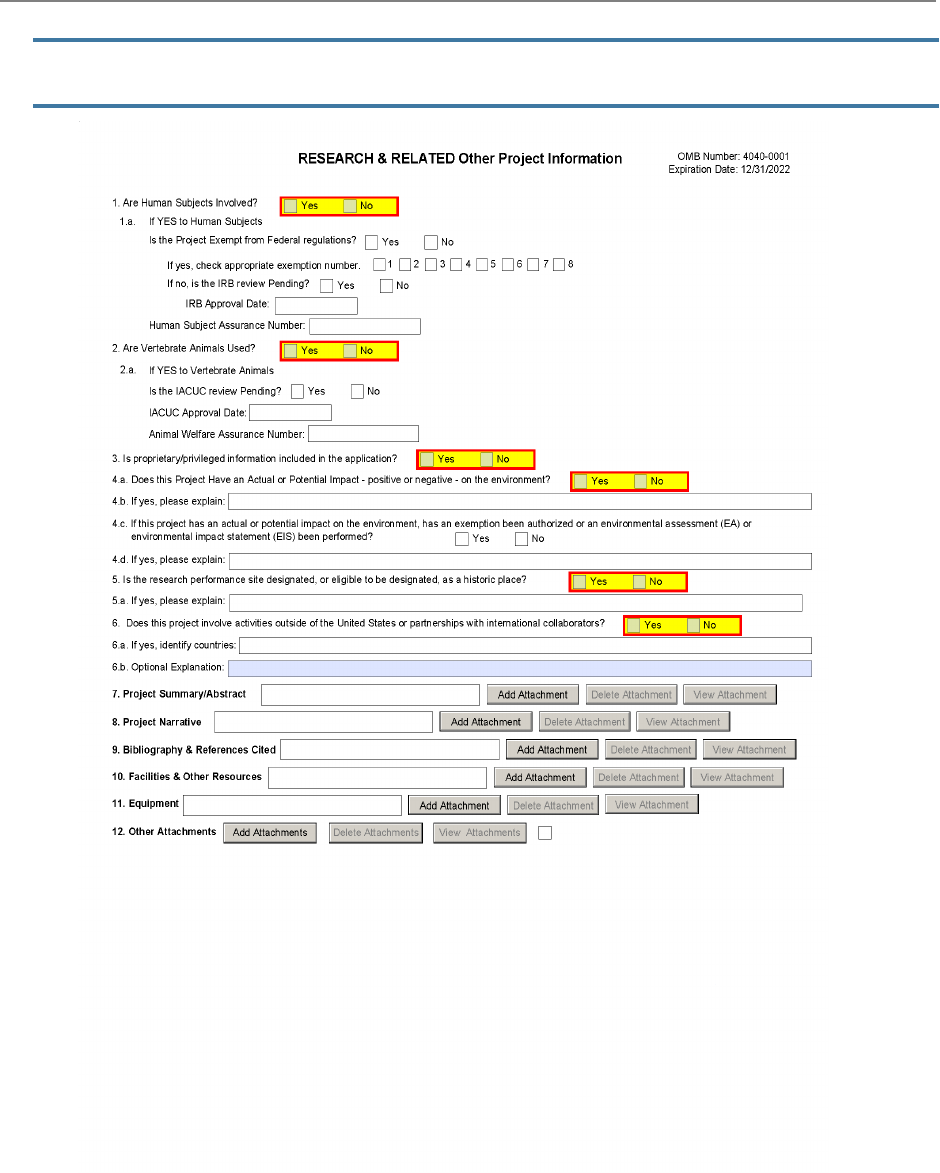
Form Screenshots
M.- vi
R&R Other Project Information Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- vii
Project/Performance Site Location(s) Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- viii
R&R Senior/Key Person Profile (Expanded) Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- ix
R&R Budget Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- x
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xi
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xii
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xiii
R&R Subaward Budget Attachment(s) Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series
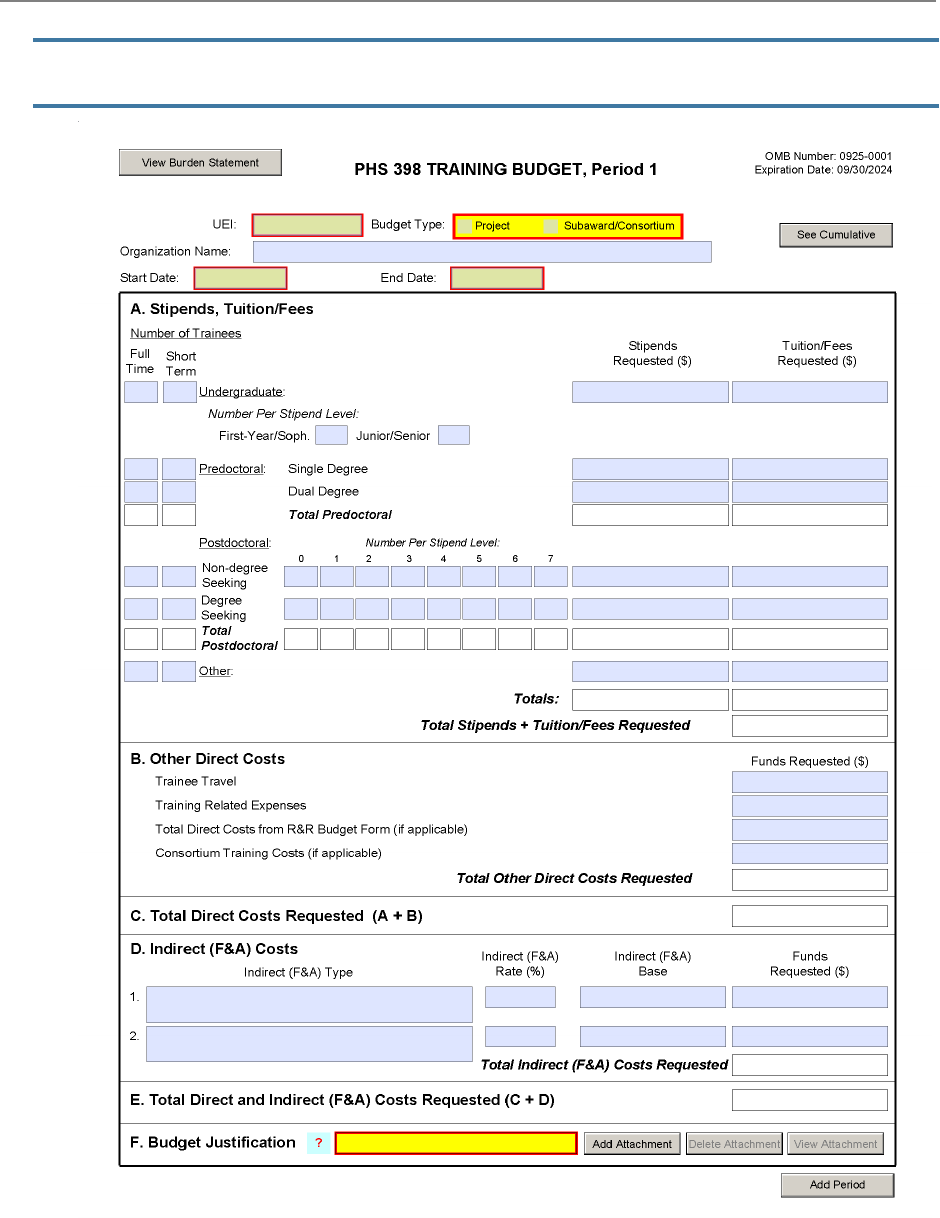
Form Screenshots
M.- xiv
PHS 398 Training Budget Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xv
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xvi
PHS 398 Training Subaward Budget Attachment(s) Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xvii
PHS Additional Indirect Cost Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xviii
PHS 398 Research Plan Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xix
PHS 398 Career Development Award Supplemental Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xx
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xxi
PHS 398 Research Training Program Plan Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xxii
PHS Human Subjects and Clinical Trials Information
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xxiii
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xxiv
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series
Form Screenshots
M.- xxv
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xxvi
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series

Form Screenshots
M.- xxvii
PHS Assignment Request Form
Multi-Project Instructions for NIH and Other PHS Agencies - Forms Version H Series
