
COVID-19
Efforts to Increase
Vaccine Availability
and Perspectives on
Initial Implementation
Report to Congressional Addressees
April 2021
GAO-21-443
United States Government Accountability Office

United States Government Accountability Office
Highlights of GAO-21-443, a report to
congressional addressees
April 2021
COVID-19
Efforts to Increase Vaccine Availability and
Perspectives
on Initial Implementation
What GAO Found
The federal government has taken several actions to increase the availability of
COVID-19 vaccine doses and indicated it expects to have enough doses
available for all adults in the United States by the end of May. As of April 1, 2021,
the government had purchased 1.2 billion doses of one- and two-dose regimen
vaccines. Also, vaccine companies reported making additional manufacturing
sites operational, among other actions to expand capacity and mitigate
challenges.
Federal officials said projecting future availability of vaccine doses can be
difficult, in part because of uncertainty surrounding complex manufacturing
processes. Given this uncertainty, coupled with the significant manufacturing and
distribution increases needed to have enough vaccine doses available for all
adults, managing public expectations is critical. GAO’s prior work has found that
timely, clear, and consistent communication about vaccine availability is essential
to ensure public confidence and trust, especially as initial vaccine implementation
did not match expectations.
COVID-19 Vaccination Site
Stakeholders GAO interviewed identified challenges with initial COVID-19
vaccine implementation. For example, some stakeholders said states often did
not have information critical to distribution at the local level, such as how many
doses they would receive and when. The federal government has begun
initiatives—outlined in a national response strategy—to improve implementation,
such as creating new vaccination sites. In its March 2021 distribution strategy,
CDC provided a high-level description of its activities and noted that more details
would be included in future reports to Congress. To meet the expectations set by
recent announcements, such as the planned expansion of vaccine eligibility to all
adults and the introduction of tools to help individuals find vaccines, it will be
imperative that the federal government effectively coordinate and communicate
its plans, as GAO recommended in September 2020.
View GAO-21-443. For more information,
contact
Alyssa M. Hundrup at (202) 512-7114
or
Why GAO Did This Study
Providing the public with safe and
effective vaccines to prevent COVID
-
19 is crucial to mitigating the public
health and economic impacts of the
disease
. The U.S. had almost 30
million reported cases and over
5
45,000 reported deaths as of March
27, 2021.
T
he federal government took
a critical step in December 2020 in
authorizing the first two COVID
-19
vaccines and beginning distribution of
doses across the nation. The
government
had distributed about
180.6
million vaccine doses, and about
147
.8 million doses had been
administered, as of
March 27, 2021,
according to
Centers for Disease
Control and Prevention
(CDC) data.
The CARES Act includes a provision
for GAO to report on its ongoing
monitoring and oversight efforts related
to the COVID
-19 pandemic. This
report
examines, among other issues, actions
the federal government has taken to
increase the availability of COVID
-19
vaccine doses, and challenges with
initial vaccine implementation
—that is,
prioritizing, allocating, distributing, and
administering vacc
ine doses—
identified by stakeholders and steps
the federal government has taken to
improve vaccine implementation.
GAO reviewed documents from the
Departments of Defense and Health
and Human Services, transcripts of
public briefings, data from
CDC, and
interviewed or received written
responses from federal officials,
vaccine company representatives, and
select public health stakeholders.
GAO
incorporated technical comments from
the Department of Defense, the
Department of Health and Human
Services, and t
he Federal Emergency
Management Agency as appropriate.

Page i GAO-21-443 COVID-19 Vaccines
Letter 1
Background 6
The Federal Government’s Contracting Approach Aimed to
Accelerate Vaccine Development and Manufacturing While
Mitigating Cost Risk to the Government 16
The Federal Government Has Purchased Additional Vaccine
Doses and Helped Mitigate Manufacturing Challenges to
Increase Vaccine Availability 24
Stakeholders Identified Challenges with Initial COVID-19
Vaccination; the Federal Government Has Taken Some Steps
to Help Improve Vaccine Implementation 32
Agency Comments 44
Appendix I GAO Contact and Staff Acknowledgments 47
Related GAO Products 48
Tables
Table 1: Status of Six COVID-19 Vaccine Candidates under the
DOD and HHS Partnership, as of March 27, 2021 8
Table 2: Manufacturing Partners for the Six COVID-19 Vaccine
Candidates under the DOD and HHS Partnership, as of
March 2021 9
Table 3: Summary of Selected Payment and Termination Terms
and Conditions Contained in the Production Awards for
the Six Operation Warp Speed Vaccine Candidates, July
2020-November 2020 19
Table 4: Contracted Amount of COVID-19 Vaccine Doses under
the Department of Defense (DOD) and Department of
Health and Human Services (HHS) Partnership, by
Vaccine Company, as of April 1, 2021 24
Table 5: Status of Manufacturing Capacity Expansion Efforts for
Six COVID-19 Vaccine Candidates under the DOD and
HHS Partnership, as of March 2021 29
Table 6: Examples of Challenges with Initial COVID-19 Vaccine
Implementation Reported by Stakeholders, as of
February 1, 2021 33
Contents

Page ii GAO-21-443 COVID-19 Vaccines
Table 7: Changes by the Federal Government to the Initial
COVID-19 Vaccine Implementation between November
2020 and mid-February 2021 35
Figures
Figure 1: Department of Defense (DOD) and Department of
Health and Human Services (HHS) Obligations for
COVID-19 Vaccine Candidates under the DOD and HHS
Partnership, as of March 14, 2021 7
Figure 2: Potential Choke Points in Scaling Up Vaccine Production
Related to Key Manufacturing Challenges 11
Figure 3: Daily Count of Doses of COVID-19 Vaccine
Administered and Reported to CDC as of March 27, 2021 15
Figure 4: Number of Doses of COVID-19 Vaccine Released in the
U.S. per Week (in millions), as of March 29, 2021 26
Figure 5: Number of Vaccine Doses Distributed in the U.S. per
Week, as of March 27, 2021 30
Abbreviations
ACIP Advisory Committee on Immunization Practices
CDC Centers for Disease Control and Prevention
COVID-19 Coronavirus Disease 2019
DOD Department of Defense
EUA emergency use authorization
FDA Food and Drug Administration
FEMA Federal Emergency Management Agency
HHS Department of Health and Human Services
National Strategy National Strategy for the COVID-19 Response and
Pandemic Preparedness
This is a work of the U.S. government and is not subject to copyright protection in the
United States. The published product may be reproduced and distributed in its entirety
without further permission from GAO. However, because this work may contain
copyrighted images or other material, permission from the copyright holder may be
necessary if you wish to reproduce this material separately.

Page 1 GAO-21-443 COVID-19 Vaccines
441 G St. N.W.
Washington, DC 20548
April 14, 2021
Congressional Addressees
Providing the public with safe and effective vaccines to prevent
Coronavirus Disease 2019 (COVID-19) is crucial to mitigating the public
health and economic impacts of the disease and ending the pandemic.
There have been almost 30 million reported cases of COVID-19 and over
545,000 reported deaths in the United States as of March 27, 2021.
1
Given this catastrophic loss of life and the pandemic’s devastating effects
on the U.S. economy, as well as new potentially harmful variants of
SARS-CoV-2, the virus that causes COVID-19, vaccines are essential for
preventing COVID-19 and related serious outcomes, such as
hospitalization or death.
In December 2020, the United States took an important step to protect
the public against COVID-19 as the first COVID-19 vaccines—developed
in a shorter time than any previous vaccine—were authorized for
emergency use and administered.
2
With three COVID-19 vaccines now
authorized for emergency use as of March 27, 2021, more than 91.7
million people had received at least one vaccine dose and more than 50.1
1
Data on COVID-19 cases in the U.S. are based on aggregate case reporting to the
Centers for Disease Control and Prevention (CDC) and include probable and confirmed
cases as reported by states and jurisdictions. CDC COVID-19 counts are subject to
change due to delays or updates in reported data from states and territories. According to
CDC, the actual number of COVID-19 cases is unknown for a variety of reasons, including
that people who have been infected may have not been tested or may not have sought
medical care. CDC’s National Center for Health Statistics COVID-19 death counts in the
U.S. are based on provisional counts from death certificate data, which do not distinguish
between laboratory-confirmed and probable COVID-19 deaths. Provisional counts are
incomplete due to an average delay of 2 weeks (a range of 1–8 weeks or longer) for death
certificate processing.
2
For more information on the accelerated COVID-19 vaccine development process, see
GAO, Operation Warp Speed: Accelerated COVID-19 Vaccine Development Status and
Efforts to Address Manufacturing Challenges. GAO-21-319, (Washington, D.C.: Feb. 11,
2021). GAO has also produced an interactive dashboard that integrates multiple data
sources to visualize the status of vaccine development, which may be found at
https://ows.gaoinnovations.gov/.
Letter

Page 2 GAO-21-443 COVID-19 Vaccines
million people had been fully vaccinated, according to Centers for
Disease Control and Prevention (CDC) data.
3
Implementing a nationwide COVID-19 vaccination program is a highly
complex undertaking involving many players. It involves multiple federal
agencies, the private sector, state, local, and territorial jurisdictions, tribal
officials, and health care providers, who must coordinate and work
together to make the vaccine available to the public.
4
At the federal level,
efforts to support vaccine development, manufacturing, and distribution to
states and other jurisdictions have been led by a partnership between the
Department of Defense (DOD) and the Department of Health and Human
Services (HHS) announced in May 2020, then known as Operation Warp
Speed. As of January 20, 2021, the federal government no longer uses
the name Operation Warp Speed, but the DOD and HHS partnership has
continued.
5
Through Operation Warp Speed and the continued DOD and HHS
partnership, the federal government has obligated at least $20 billion as
of March 14, 2021, mostly through awards to six vaccine companies for
COVID-19 vaccine candidates, with various development and
manufacturing activities associated with these awards. Initial awards
made from March 2020 through June 2020 were generally to fund
vaccine development efforts, including clinical trials, and later awards
made from July 2020 through December 2020 were generally for vaccine
manufacturing or the purchase of vaccine doses. Since then, the
3
Two of the COVID-19 vaccines being administered as of this date were two-dose vaccine
regimens and one was a single dose. According to CDC, the count of people receiving at
least one dose represents the total number of people who received at least one dose of a
COVID-19 vaccine, including those who received the single-dose vaccine. The count of
fully vaccinated people represents the number of people who have received the second
dose of a two-dose COVID-19 vaccine regimen and those who received the single-dose
COVID-19 vaccine.
4
For COVID-19 vaccination there are 64 jurisdictions including all 50 states, territories,
and local health programs in Chicago, the District of Columbia, Houston, New York City,
Philadelphia, and San Antonio.
5
DOD and HHS’s partnership is to continue through May 1, 2021, per an extension of a
memorandum of understanding between the two departments. According to officials
working under Operation Warp Speed and the continued partnership, the leadership
structure is generally the same, but personnel in some key senior leadership positions
have changed. In this report, we use “Operation Warp Speed” to reference the actions the
partnership had taken while it was operating under that name and refer to the “DOD and
HHS partnership” for actions after January 20, 2021.

Page 3 GAO-21-443 COVID-19 Vaccines
government has purchased additional vaccine doses through exercising
options on previously awarded contracts.
Since June 2020, we have cited the critical importance of planning for the
development, manufacturing, distribution, and administration of COVID-
19 vaccines.
6
We recommended, in September 2020, that the Secretary
of Health and Human Services, with support from the Secretary of
Defense, establish a time frame for documenting and sharing a national
plan for distributing and administering COVID-19 vaccines, ensure that
such a plan is consistent with best practices for project planning and
scheduling, and ensure that the plan outlines an approach for how efforts
would be coordinated across federal agencies and nonfederal entities.
We also have noted the importance of timely, clear, and consistent
communication to stakeholders like state, territorial, and local health
officials and health care providers, as well as to the public about vaccine
availability, effectiveness, and safety to help ensure public confidence
and trust, which in turn could encourage vaccine use. In January 2021,
we reported that initial vaccine implementation did not match
expectations, and we reiterated the vital importance of federal planning,
leadership, and coordination.
The CARES Act includes a provision for GAO to report on its ongoing
monitoring and oversight efforts related to the COVID-19 pandemic.
7
This
report is part of our body of work in response to the CARES Act and
focuses on the federal government’s efforts related to COVID-19
vaccines. Specifically, in this report, we describe
(1) how the initial awards made under Operation Warp Speed address
specific terms and conditions, including government rights to intellectual
property;
(2) actions the federal government has taken to increase the availability of
COVID-19 vaccine doses;
6
We have released a series of products on the federal government’s response to COVID-
19 since June 2020, including the government’s efforts related to COVID-19 vaccines and
therapeutics. See the related products section of this report for a list of those products.
7
Pub. L. No. 116-136, § 19010, 134 Stat. 281, 579-81 (2020). This report also responds,
in part, to a bipartisan request from the House Select Subcommittee on the Coronavirus
Crisis for GAO to examine Operation Warp Speed.

Page 4 GAO-21-443 COVID-19 Vaccines
(3) challenges with initial vaccine implementation—that is, prioritizing,
allocating, distributing, and administering vaccine doses—identified by
stakeholders and steps the federal government has taken to improve
vaccine implementation.
To describe how the awards made under Operation Warp Speed address
specific terms and conditions—such as pricing, payment, termination
terms and conditions, and government rights to intellectual property and
data—we reviewed DOD and HHS acquisition documents related to
Operation Warp Speed, including award documents, statements of work,
and related amendments for the six vaccine candidates for awards made
from March 2020 through November 2020.
8
We reviewed data reported
by Federal Procurement Data System-Next Generation through March
14, 2021 and agreements from DOD, HHS, and Advanced Technology
International.
9
All award amounts are based on Federal Procurement
Data System–Next Generation obligations data. We assessed the
reliability of data reported to the Federal Procurement Data System-Next
Generation by performing electronic testing and by comparing this
information to the contract documents we obtained. We determined that
the data were sufficiently reliable for the purposes of describing agencies’
reported contract obligations for the six vaccine candidates and other
vaccine-related obligations. In addition, we interviewed DOD and HHS
officials and coordinated with all six vaccine companies to better
understand their perspectives on the intellectual property provisions,
termination and payment terms and conditions, and the implications of the
foregoing.
To describe actions the federal government has taken to increase the
availability of COVID-19 vaccines doses, we reviewed information and
data from Operation Warp Speed and the continued DOD and HHS
partnership on the purchase, manufacture, and release of COVID-19
vaccine doses from July 2020 through March 2021.
10
We also reviewed
8
The six companies with COVID-19 vaccine candidates are AstraZeneca, Janssen,
Moderna, Novavax, Pfizer/BioNTech, and Sanofi/GSK. For the purpose of this report, we
refer to the Pfizer/BioNTech collaboration as “Pfizer” and to the Sanofi/GSK collaboration
as “Sanofi.” Janssen Pharmaceutical Companies are a part of Johnson & Johnson.
9
Advanced Technology International manages the Medical Chemical, Biological,
Radiological and Nuclear Defense Consortium, a partnership with industry, academic, and
not-for-profit partners to support the DOD’s medical, pharmaceutical, and diagnostic
requirements.
10
According to DOD and HHS partnership officials, once through manufacturing and
quality assurance, the vaccine doses are released for distribution.

Page 5 GAO-21-443 COVID-19 Vaccines
CDC data on vaccine doses distributed to jurisdictions and federal entities
as of March 27, 2021. We also interviewed or received written responses
from the six companies with COVID-19 vaccine candidates and DOD,
HHS, and CDC officials about the data and the actions they had taken to
increase the availability of vaccines. We assessed the reliability of the
data from CDC and the DOD and HHS partnership by reviewing relevant
CDC documentation, such as documentation that defines data points,
including any caveats, and interviewing DOD and HHS partnership
officials about these data. We determined that these data were sufficiently
reliable for the purposes of our reporting objective.
To describe challenges with initial vaccine implementation—that is,
prioritizing, allocating, distributing, and administering vaccine doses—
identified by stakeholders and steps the federal government has taken to
improve vaccine implementation, we interviewed representatives of state,
territorial, and local health officials and health care providers who are
involved in vaccine distribution and administration efforts.
11
We also
reviewed documentation, such as recommendations published by CDC’s
Advisory Committee on Immunization Practices (ACIP), the White
House’s National Strategy for the COVID-19 Response and Pandemic
Preparedness (National Strategy) released in January 2021, White House
fact sheets issued in January and February 2021 related to vaccine
distribution, CDC’s COVID-19 Vaccination Program Interim Playbook for
Jurisdictions Operations Annex, CDC vaccine allocation data, and
vaccine administration data from CDC’s COVID Data Tracker website,
11
For perspectives of state, territorial, and local health officials, we reviewed documents
from and interviewed representatives from the Association of State and Territorial Health
Officials, Association of Immunization Managers, American Immunization Registry
Association, and the National Association of County and City Health Officials. We also
interviewed one public health infectious disease specialist who also serves as the Health
Officer for Public Health for a county that includes a major metropolitan area. We also
interviewed officials from the National Governors Association.
For perspectives of health care providers, we interviewed representatives from the
American Hospital Association, American Medical Association, American Nurses
Association, American College of Emergency Physicians, America’s Essential Hospitals,
Association for Health Care Resource & Materials Management, American Public Health
Association, and the National Association of Community Health Centers.

Page 6 GAO-21-443 COVID-19 Vaccines
among others.
12
In addition, we interviewed and reviewed written
responses from HHS and DOD officials, including officials from their
partnership and CDC, and reviewed documentation such as transcripts
from briefings during which the Secretary of Health and Human Services
or other federal officials provided information to the public about vaccine
distribution and administration efforts.
We conducted this performance audit from October 2020 to April 2021 in
accordance with generally accepted government auditing standards.
Those standards require that we plan and perform the audit to obtain
sufficient, appropriate evidence to provide a reasonable basis for our
findings and conclusions based on our audit objectives. We believe that
the evidence obtained provides a reasonable basis for our findings and
conclusions based on our audit objectives.
To accelerate the availability of a vaccine to prevent COVID-19, DOD, on
behalf of HHS, awarded contracts and other transaction agreements
(referred to in this report as “agreements”) in 2020 to six vaccine
companies for different types of activities.
13
Operation Warp Speed
officials indicated in August 2020 that they selected the six vaccine
candidates from three vaccine-platform technologies that they considered
to be the most likely to quickly yield a safe and effective vaccine.
14
As of March 14, 2021, DOD and HHS had obligated at least $20 billion to
develop, manufacture, track, and distribute vaccines for COVID-19 under
12
See White House, National Strategy for the COVID-19 Response and Pandemic
Preparedness (Washington, D.C.: Jan. 21, 2021), accessed on January 21, 2021 at
https://www.whitehouse.gov/wp-content/uploads/2021/01/National-Strategy-for-the-
COVID-19-Response-and-Pandemic-Preparedness.pdf and Department of Health and
Human Services, Centers for Disease Control and Prevention, COVID-19 Vaccination
Program Interim Playbook for Jurisdictions Operations Annex (Atlanta, Ga.: Jan. 11,
2021), accessed on February 4, 2021, https://www.cdc.gov/vaccines/covid-
19/downloads/COVID-19-vaccination-program-playbook-annex.pdf. For the CDC data
tracker see https://covid.cdc.gov/covid-data-tracker/#vaccinations (accessed Mar. 1,
2021).
13
For example, for some vaccine candidates, Operation Warp Speed and the continued
DOD and HHS partnership publicly announced support for both clinical development and
manufacturing activities; while for some candidates, it only announced support for the
manufacturing or purchase of vaccine doses.
14
For more information on the characteristics and development status of the individual
Operation Warp Speed vaccine candidates, see GAO-21-319.
Background
DOD and HHS
Partnership and COVID-
19 Vaccines

Page 7 GAO-21-443 COVID-19 Vaccines
Operation Warp Speed and the continued DOD and HHS partnership, as
shown in figure 1 below.
Figure 1: Department of Defense (DOD) and Department of Health and Human Services (HHS) Obligations for COVID-19
Vaccine Candidates under the DOD and HHS Partnership, as of March 14, 2021
Note: The DOD and HHS partnership was formerly known as Operation Warp Speed. We
used the HHS Operation Warp Speed website and HHS press releases to determine
which contract obligations to include in our analysis. For some vaccine candidates,
Operation Warp Speed and the continued DOD and HHS partnership publicly announced
support for both clinical development and manufacturing activities; while for some
candidates, it only announced support for the manufacturing or purchase of vaccine
doses. HHS announced two awards related to distribution for which we could not identify
obligations in the Federal Procurement Data System-Next Generation, which are not
included in the chart above.
Typically, before a vaccine can be marketed in the United States, it must
be licensed by the Food and Drug Administration (FDA).
15
An emergency
15
FDA—an agency within HHS—licenses biological products, including vaccines, through
review of a biologics license application.

Page 8 GAO-21-443 COVID-19 Vaccines
use authorization (EUA) allows for the temporary use of vaccines without
FDA licensure, provided certain statutory criteria are met.
16
As of March 27, 2021, FDA had issued EUAs for three COVID-19
vaccines: (1) Pfizer’s COVID-19 vaccine on December 11, 2020; (2)
Moderna’s COVID-19 vaccine on December 18, 2020, and (3) Janssen’s
COVID-19 vaccine on February 27, 2021.
17
There were no FDA-licensed
COVID-19 vaccines, as of March 27, 2021.
18
See table 1 for the status of
each of the six vaccine candidates under the DOD and HHS partnership,
as of March 27, 2021.
Table 1: Status of Six COVID-19 Vaccine Candidates under the DOD and HHS Partnership, as of March 27, 2021
Vaccine company
Started phase 3
clinical trials
a
Announced initial
findings from phase
3 clinical trials
Submitted
emergency use
authorization (EUA)
request to FDA
b
FDA issued EUA
Pfizer
●
●
●
●
Moderna
●
●
●
●
Janssen
c
●
●
●
●
AstraZeneca
●
●
16
The Secretary of Health and Human Services may declare that circumstances,
prescribed by statute, exist justifying the emergency use of certain medical products, such
as vaccines. Once a declaration of an emergency has been made, FDA may temporarily
allow use of unlicensed vaccines through an EUA. For FDA to issue an EUA for a vaccine,
it must be reasonable to believe that the vaccine may be effective and that the known and
potential benefits of the vaccine outweigh the known and potential risks, among other
statutory criteria. See 21 U.S.C. § 360bbb-3.
17
On February 4, 2021, FDA announced that it was assessing the impact of new SARS-
CoV-2 strains on authorized vaccines, and was working with companies and international
partners to evaluate the impact that each variant may have on the effectiveness of
authorized vaccines. FDA also stated that at the time, available information suggested that
the authorized vaccines remained effective in protecting the American public against
currently circulating strains of COVID-19. On February 22, 2021, FDA released an
updated version of its October 2020 guidance, Emergency Use Authorization for Vaccines
to Prevent COVID-19, to provide recommendations to companies seeking to amend their
EUAs for COVID-19 vaccines to address new variants.
On Tuesday, April 13, 2021, CDC and FDA released a joint statement recommending a
pause in the use of Janssen vaccine while CDC and FDA review data involving six
reported U.S. cases of a rare and severe type of blood clot in individuals after receiving
this vaccine. CDC and FDA’s announcement occurred after we finalized our analyses for
this report, but we will continue to monitor this in our future work.
18
Any COVID-19 vaccine that initially receives an EUA from FDA is expected to ultimately
be reviewed and receive licensure through a biologics license application, according to
FDA guidance.

Page 9 GAO-21-443 COVID-19 Vaccines
Vaccine company
Started phase 3
clinical trials
a
Announced initial
findings from phase
3 clinical trials
Submitted
emergency use
authorization (EUA)
request to FDA
b
FDA issued EUA
Novavax
●
●
d
Sanofi
e
Source: GAO analysis of vaccine company, Operation Warp Speed, and Food and Drug Administration (FDA) information. | GAO-21-443
Note: The partnership between the Department of Defense (DOD) and the Department of Health and
Human Services (HHS) was formerly known as Operation Warp Speed.
a
Phase 3 clinical trials are performed after preliminary evidence suggesting effectiveness of a product
has been obtained, and are intended to gather additional information about safety and effectiveness.
These trials involve several hundred to thousands of volunteers, usually including participants who
are at increased risk for infection. Earlier phases generally involve fewer volunteers and test issues
such as safety of the product (phase 1) and the effectiveness of the product for a particular use and
additional safety information (phase 2).
b
During an emergency, as declared by the Secretary of Health and Human Services under 21 U.S.C.
§ 360bbb-3(b), FDA may temporarily authorize unlicensed vaccines through an EUA, provided certain
statutory criteria are met. FDA has indicated that issuance of an EUA for a COVID-19 vaccine for
which there is adequate manufacturing information would require a determination by FDA that the
vaccines benefits outweigh its risks based on data from at least one well-designed phase 3 clinical
trial that demonstrates the vaccine’s safety and efficacy in a clear and compelling manner. Any
COVID-19 vaccine that initially receives an EUA from FDA is expected to ultimately be reviewed and
receive licensure through a biologics license application, according to FDA guidance.
c
Janssen Pharmaceutical Companies are a part of Johnson & Johnson.
d
Novavax has announced findings from its final analysis of phase 3 clinical trial data from the United
Kingdom. As of March 27, 2021, it had not yet announced findings based on its phase 3 clinical trial in
the United States.
e
Sanofi announced in December 2020 that global phase 3 clinical trials could start during the second
quarter of 2021; pending positive data from a phase 2 study expected to start in February 2021. On
February 22, 2021, Sanofi announced the start of its phase 2 study.
The six vaccine companies with candidates under the DOD and HHS
partnership are working with different manufacturing partners, as of
March 2021, to make use of available manufacturing capacity, as shown
in table 2. These U.S. manufacturing partners are located at sites across
the country.
Table 2: Manufacturing Partners for the Six COVID-19 Vaccine Candidates under the
DOD and HHS Partnership, as of March 2021
Vaccine company
Drug substance
manufacturing partners for
U.S. market
a
Fill-Finish manufacturing
partners for U.S. market
b
Pfizer
All manufacturing is currently
being conducted by Pfizer
All manufacturing is currently
being conducted by Pfizer
Moderna
Lonza
Baxter BioPharma Solutions
and Catalent
COVID-19 Vaccine
Manufacturing and
Related Challenges

Page 10 GAO-21-443 COVID-19 Vaccines
Vaccine company
Drug substance
manufacturing partners for
U.S. market
a
Fill-Finish manufacturing
partners for U.S. market
b
Janssen
Emergent BioSolutions and
Merck
c
Catalent, Grand River Aseptic
Manufacturing, Merck
c
, and PCI
Pharma Services
AstraZeneca
Emergent BioSolutions
AMRI and PCI Pharma Services
Novavax
AGC Biologics and Fuji
Diosynth Biotechnologies
Jubilant HollisterStier and Par
Sterile Products
Sanofi
Fuji Diosynth Biotechnologies
Sanofi has not yet begun fill-
finish manufacturing for its
vaccine candidate
Source: GAO analysis of information from vaccine companies. | GAO-21-443.
Note: The Department of Defense (DOD) and Department of Health and Human Services (HHS)
partnership was formerly known as Operation Warp Speed.
a
Drug substance manufacturing is the production of bulk amounts of the vaccine drug substance.
b
Fill-finish manufacturing is the transfer of vaccine into sterile containers.
c
As of March 2021, the Merck drug substance and fill-finish facilities had not yet been activated and
will not be used to produce any of the initial 100 million COVID-19 vaccine doses that Janssen
provides to the U.S. government, according to a Janssen representative.
As we reported in November 2020 and February 2021, the federal
government is working with vaccine companies to help mitigate
challenges that could hinder vaccine manufacturing, including:
(1) limited manufacturing capacity,
(2) disruption to supply chains,
(3) difficult technology transfer processes, and
(4) gaps in workforce availability.
19
There are a number of potential manufacturing choke points that could
result from those manufacturing challenges, as shown in figure 2 below.
For example, the ability to hire and train personnel with the specialized
skills needed to run vaccine manufacturing processes can be a challenge
for even experienced manufacturers. We heard from representatives at a
facility manufacturing COVID-19 vaccines that filling open positions for
mid- to upper management had been a challenge. These positions are
19
GAO-21-319; GAO, COVID-19: Federal Efforts Accelerate Vaccine and Therapeutic
Development, but More Transparency Needed on Emergency Use Authorizations,
GAO-21-207 (Washington, D.C.: Nov. 17, 2020).

Page 11 GAO-21-443 COVID-19 Vaccines
significant because manufacturing managers function as the technical
points of contact for production questions and are responsible for
managing safety, quality, and compliance with current good
manufacturing practices.
Figure 2: Potential Choke Points in Scaling Up Vaccine Production Related to Key Manufacturing Challenges

Page 12 GAO-21-443 COVID-19 Vaccines
Vaccine implementation, following authorization or licensure, involves
several key steps (which may happen concurrently) and various
stakeholders. The stakeholders include several federal agencies and
multiple state and local stakeholders, including the DOD and HHS
partnership, CDC, private industry, jurisdictions, local health departments,
tribal officials, and health care providers.
• Prioritization. ACIP issues recommendations to the CDC Director for
target groups to receive initial vaccine doses based on its review of
data, including vaccine safety and efficacy data.
20
These
recommendations as adopted are not binding on jurisdictions, and
jurisdictions can adopt different approaches. In addition, the Secretary
of Health and Human Services may issue directives regarding
prioritization and eligibility for COVID-19 vaccinations, according to
HHS.
• Allocation. The DOD and HHS partnership determines the amount of
COVID-19 vaccine allocated for 64 jurisdictions, which include all U.S.
states, territories, the District of Columbia and local health programs
in Chicago, Houston, New York City, Philadelphia, and San Antonio.
21
According to DOD and HHS partnership officials, allocations are
based on each jurisdiction’s adult population. The partnership also
allocates additional doses to jurisdictions for American Indian/Alaskan
Native populations that elected to receive vaccines through the state
20
Within priority groups are separate target groups that are added as vaccine supply
increases. For example, ACIP recommended that when vaccine supplies are not sufficient
to meet demand, vaccine should be offered in phases. In December 2020, ACIP
recommended that phase 1 include the target groups of 1a (residents of long-term care
facilities and health care workers), 1b (persons 75 years and older and frontline essential
workers), and 1c (persons aged 65-74 years and persons aged 16-64 years with medical
conditions that increase the risk for severe COVID-19). ACIP is comprised of medical and
public health experts who make recommendations on the use of vaccines in the civilian
population of the United States. Its recommendations serve as public health guidance for
safe use of vaccines and other related products. If adopted by the CDC Director, ACIP’s
recommendations are published as official HHS/CDC recommendations in the Morbidity
and Mortality Weekly Report. See COVID-19 ACIP Vaccine Recommendations, accessed
March 9, 2021, https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html.
21
Included in the vaccines allocated to the jurisdictions were vaccine doses for the federal
government’s Pharmacy Partnership for Long-Term Care Program, which has facilitated
on-site vaccination of residents and staff at long-term care facilities. In addition to the
vaccine doses allocated to the 64 jurisdictions, the DOD and HHS partnership directly
allocates vaccine doses to five federal entities (the Bureau of Prisons, DOD, Department
of State, Indian Health Service, and Veterans Health Administration).
Implementation of COVID-
19 Vaccines

Page 13 GAO-21-443 COVID-19 Vaccines
instead of through the Indian Health Service.
22
According to HHS
data, 10 jurisdictions are receiving these additional doses—called
“Sovereign Nation Supplements” by the DOD and HHS partnership—
in their allocations.
In addition, vaccine doses are also being made available through
other federal efforts. For example, in February 2021, the
administration announced that vaccine doses would go to specific
mass vaccination clinics, pharmacy partnerships, and federally
qualified health centers. These allocations are in addition to the
jurisdiction and federal entity allocations.
• Distribution. Health care providers receiving COVID-19 vaccine
doses from their jurisdictions’ allocations place orders for vaccines
that the jurisdictions review and approve.
23
Vaccine doses and
ancillary supplies (such as syringes) are distributed to jurisdictions
and health care providers from a central distributor, except for Pfizer’s
vaccine doses, which are shipped to jurisdictions and health care
providers directly from the manufacturer. According to CDC, vaccine
doses are considered “delivered” when the jurisdictions and other
sites receive them.
24
• Administration. Health care providers administer vaccines at
administration sites, including pharmacies, hospitals, long-term care
facilities, federally qualified health centers, rural health centers,
physician offices, colleges and universities, and mass-vaccination
22
Tribal health programs and Urban Indian Organizations can decide to receive vaccines
either through the Indian Health Service or through the jurisdiction in which they are
located.
23
The maximum number of doses a jurisdiction can approve for order is an order cap set
for each jurisdiction based on the allocated amount. When the number of doses ordered
by health care providers exceeds the jurisdiction’s order cap, not all orders will be
approved.
24
Where vaccines are delivered within a jurisdiction can vary and depends on what
jurisdictions have specified in their vaccine implementation planning. Moderna’s vaccine is
delivered in lot sizes of 100 doses, while the Pfizer vaccine is delivered in lot sizes of
1,170 doses as of February 16, 2021. In some cases, vaccines are delivered to a
centralized site within a jurisdiction and then further subdivided into smaller lot sizes for
delivery to additional sites, such as facilities in rural areas, for administration; in other
cases, vaccines are directly delivered to sites for administration, such as to large health
care systems that have the needed storage and handling capability, according to CDC.

Page 14 GAO-21-443 COVID-19 Vaccines
clinics.
25
After administration, health care providers must record the
vaccination data for each individual vaccinated in the appropriate data
systems for their jurisdiction within 72 hours, according to CDC.
26
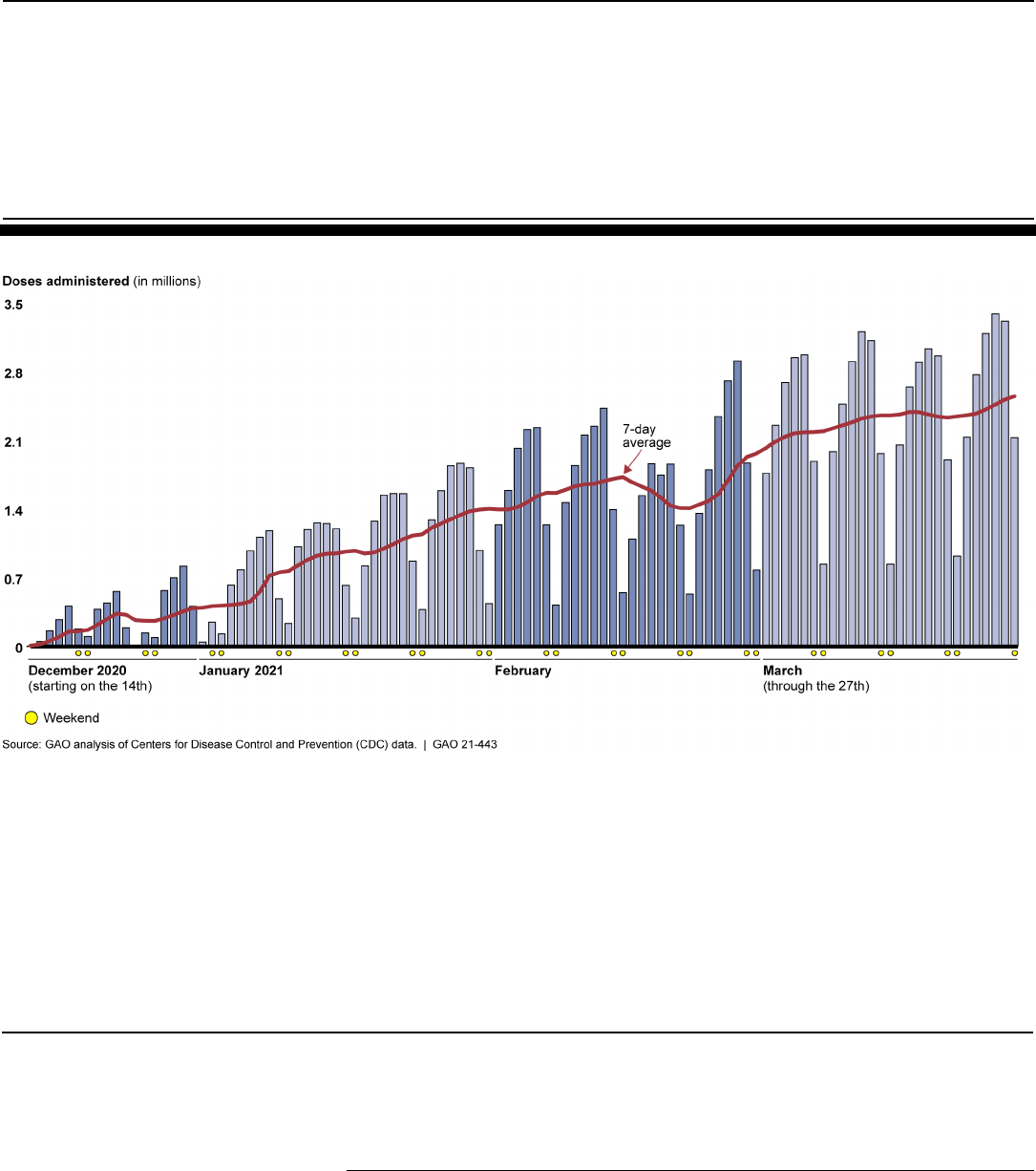
As of March 27, 2021, CDC data showed the federal government had
distributed about 180.6 million doses of COVID-19 vaccine, and health
care providers reported administering about 147.8 million COVID-19
vaccine doses, which includes both first and second doses
administered.
27
See figure 3 below for the number of doses reported to
CDC as administered, for each day from mid-December 2020, when
vaccine doses were first administered, through March 27, 2021.
25
Only those providers participating in the CDC COVID-19 Vaccination Program may
administer COVID-19 vaccines. These providers must sign a CDC COVID-19 Vaccination
Program Provider Agreement and adhere to all requirements outlined in the agreement.
Federally qualified health centers operate as part of the Health Center Program
administered by the Bureau of Primary Health Care within the Health Resources and
Services Administration. The Health Center Program provides grants to federally qualified
health centers under section 330 of the Public Health Service Act. See 42 U.S.C. § 254b.
26
For patients administered a vaccine requiring two doses, health care providers should
use redundant methods and systems, such as phone calls, emails, or text messages to
remind patients to obtain their second dose, according to CDC’s COVID-19 Vaccination
Program Interim Playbook for Jurisdiction Operations.
27
Vaccine doses distributed is the total number that have been delivered to jurisdictions,
retail pharmacies, long-term care facilities, Federal Emergency Management Agency and
Health Resources and Services Administration partner sites, and federal entities.
However, for Palau, Micronesia, Marshall Islands, Guam, American Samoa, and Northern
Marianas Islands, total counts of COVID-19 vaccine doses distributed include doses
marked as shipped in CDC’s Vaccine Tracking System since December 13, 2020.
Page 15 GAO-21-443 COVID-19 Vaccines
Figure 3: Daily Count of Doses of COVID-19 Vaccine Administered and Reported to CDC as of March 27, 2021
Note: Data show the number of COVID-19 vaccine doses administered in the U.S. as reported to
CDC by state, territorial, and local public health agencies, and federal entities, since the national
vaccine program began on December 14, 2020, and include doses administered through all vaccine
partners including jurisdictional partner clinics, retail pharmacies, long-term care facilities, Federal
Emergency Management Agency and Health Resources and Services Administration partner sites,
and federal entity facilities. The data were accessed April 1, 2021. As of March 27, 2021, three
COVID-19 vaccines were authorized for emergency use; two of these vaccines are two-dose
regimens and the third vaccine requires one dose. The number of doses administered on a given day
may be affected by several factors, such as weekend days, holidays, weather, and vaccine
availability. On February 19, 2021, officials from the White House COVID-19 Response Team said
severe weather across the country impacted vaccine distribution and administration in all 50 states.
Further, officials said the shipment of 3 days’ worth of vaccine doses—about 6 million doses—was
delayed due to weather.
On January 21, 2021, the White House issued its National Strategy for
COVID-19 Response and Pandemic Preparedness.
28
The National
Strategy identifies seven goals for a coordinated response to address the
COVID-19 pandemic and outlines several ongoing and new actions to
28
White House, National Strategy for the COVID-19 Response and Pandemic
Preparedness (Washington, D.C.: Jan. 21, 2021), accessed on January 21, 2021 at
https://www.whitehouse.gov/wp-content/uploads/2021/01/National-Strategy-for-the-
COVID-19-Response-and-Pandemic-Preparedness.pdf.
National Strategy for
COVID-19 Response and
Pandemic Preparedness

Page 16 GAO-21-443 COVID-19 Vaccines
help achieve the administration’s goals.
29
The National Strategy states
that, among other things, the federal government will ensure the
availability of safe, effective vaccines for the American public through
actions to purchase and manufacture doses sufficient to vaccinate the
U.S. population.
From March to November 2020, the federal government awarded
contracts and agreements to six vaccine companies to accelerate the
development of safe and effective COVID-19 vaccines while balancing
the risk to the government in doing so. Specifically, during this time, the
federal government made awards for vaccine development, manufacture,
or purchase of an initial 600 million doses with an estimated value of
$12.8 billion.
30
These awards were made, according to DOD and HHS
partnership officials, in anticipation that some of the vaccine candidates
would subsequently receive authorization or licensure. By providing
significant funding up front, these officials told us, the government took on
some financial risk, which enabled the companies to accelerate vaccine
development and production. However, the government also incorporated
safeguards in the contracts and agreements to mitigate its financial risk,
by including, for example, payment and termination language intended to
limit the government’s liability if a vaccine candidate is not authorized or
licensed.
31
In effect, according to DOD officials, the government aimed to
balance financial risks and help ensure that the government would
29
The seven goals are: (1) restore trust with the American people; (2) mount a safe,
effective, and comprehensive vaccination campaign; (3) mitigate spread through
expanding masking, testing, data, treatments, health care workforce, and clear public
health standards; (4) immediately expand emergency relief and exercise the Defense
Production Act; (5) safely reopen schools, businesses, and travel while protecting
workers; (6) protect those most at risk and advance equity, including across racial, ethnic,
and rural/urban lines; and (7) restore U.S. leadership globally and build better
preparedness for future threats.
30
From March to April 2020 before Operation Warp Speed was formally established, HHS
made awards to three companies—Janssen, Moderna and Sanofi—for development
and/or clinical studies. After Operation Warp Speed was established in May 2020, DOD
awarded additional contracts and agreements for vaccine manufacturing. We refer to all
these awards as part of the Operation Warp Speed effort. The production awards allow
the government to purchase additional doses through options or follow-on awards. In
October, DOD made an additional award to AstraZeneca for 200 million doses. The Pfizer
and Moderna vaccines received EUAs on December 11, 2020 and December 18, 2020
respectively, and the federal government awarded an additional contract and exercised a
contract option for an additional 200 million doses from Pfizer, and exercised options for
an additional 200 million doses from Moderna.
31
According to DOD officials, another risk mitigation strategy included negotiating a
requirement for domestic, large-scale vaccine manufacturing, which was intended to avoid
manufacturing-related risks and better ensure timely delivery.
The Federal
Government’s
Contracting Approach
Aimed to Accelerate
Vaccine Development
and Manufacturing
While Mitigating Cost
Risk to the
Government

Page 17 GAO-21-443 COVID-19 Vaccines
receive a sufficient number of vaccine doses, even if one or more
companies’ efforts failed to produce a viable vaccine.
DOD and HHS officials told us that they took a different contracting
approach with each of the six vaccine companies as each came to the
negotiating table with differing levels of product maturity and
manufacturing capability. For example, with the exception of Pfizer, the
federal government provided funding to five companies for some level of
vaccine development including for activities such as preclinical and
clinical trials. According to DOD and HHS partnership officials, this
funding gave the government insight into vaccine development or
manufacturing that it did not have with Pfizer. For example, according to
these officials, the five other vaccine companies agreed to allow DOD and
HHS partnership officials in their manufacturing facilities to provide in-
depth visibility into production capabilities and any challenges that may
arise.
Our review of awards made to the six Operation Warp Speed vaccine
candidates through November 2020 found that the specific terms and
conditions the federal government negotiated with the companies varied.
For example:
• Pursuant to Pfizer’s July 2020 agreement with the U.S. government,
which awarded Pfizer $1.95 billion, the government agreed to pay a
firm-fixed-price of $19.50 per dose for the first 100 million doses of the
Pfizer COVID-19 vaccine. To minimize financial risk to the
government, the parties agreed that the government would pay Pfizer
only after its vaccine received authorization or licensure from FDA and
as the doses were delivered. Further, Pfizer and the government
agreed that if Pfizer should cease to develop the vaccine due to
emerging safety or efficacy data, the government would be able to
terminate the July 2020 agreement and put into effect a no-cost
settlement to end the performance under the agreement within 30
days of notifying the company.
• In April 2020, HHS awarded an agreement with Moderna for $430
million for clinical trials and other aspects of vaccine development,
which included providing the government insight into the vaccine
candidate’s progress toward viability.
32
Subsequently, in August 2020,
32
The agreement also included manufacturing scale up and validation of manufacturing
capacities.

Page 18 GAO-21-443 COVID-19 Vaccines
DOD awarded Moderna a production contract and obligated $1.2
billion to manufacture 100 million doses at a price of $12.25 per dose
with options to buy 400 million more.
33
DOD agreed to pay Moderna
incrementally for meeting certain milestones without requiring
Moderna to first obtain an EUA or licensure.
34
In the event that
Moderna failed to obtain an EUA or licensure, DOD would be able to
terminate the contract in whole or in part. According to Operation
Warp Speed officials, this approach assisted the company with cash
flow by providing interim payments. Further, the government could
unilaterally decide that it would not exercise any of the four options
included in the award, which provided for 100 million doses each at a
cost of $16.50 per dose.
For the other four vaccine companies, the federal government provided
different levels of support for vaccine development, but DOD negotiated
similar payment and termination terms and conditions in their production
agreements. These payment and termination terms and conditions
enabled the government to adjust its reliance on the various companies
as time progressed and more was known about their vaccine candidates,
according to DOD officials we interviewed.
DOD’s approach to event-based terminations and termination in the
federal government’s best interests is similar to the approach used in
most federal contracts. For example, DOD could terminate its agreements
with AstraZeneca or Janssen for an event-based reason such as if FDA
placed clinical trials on hold for a certain amount of time (AstraZeneca) or
revoked an existing EUA (both companies).
35
DOD could also terminate
its agreements with several companies if it determined that doing so was
in the government’s interests. The government would then pay the
relevant company for work performed in accordance with the agreement
terms. Table 3 summarizes the selected payment and termination terms
33
While the contract has a firm-fixed price of $12.25 per dose for the first 100 million
doses, it also included an incentive of $3.00 per dose for meeting an EUA deadline of
January 31, 2021. As Moderna’s vaccine received an EUA on December 18, 2020, the
company is eligible to receive the additional incentive payment.
34
See Federal Acquisition Regulation § 52.232-32 (Performance-Based Payments).
35
FDA may revise or revoke an EUA if the circumstances giving rise to the emergency
declaration no longer exist, the statutory criteria for issuance are no longer met, or
revocation is appropriate to protect public health or safety. For example, an EUA may be
revoked if new data become available indicating that the vaccine is not safe or effective. In
general, unless it is revoked, an EUA will remain in effect for the duration of the
emergency declaration. See 21 U.S.C. § 360bbb-3(f) and (g).

Page 19 GAO-21-443 COVID-19 Vaccines
and conditions that were included in the vaccine agreements we reviewed
in the stated timeframe.
Table 3: Summary of Selected Payment and Termination Terms and Conditions Contained in the Production Awards for the
Six Operation Warp Speed Vaccine Candidates, July 2020-November 2020
Company/initial award
date/value/quantity
Payment Termination
Pfizer
July 2020
$1.95 billion
100 million doses
No payment until biologics license
application (BLA) or emergency use
authorization (EUA) obtained and as
doses are delivered pursuant to the
agreement.
a
If Pfizer ceases to develop the vaccine for reasons
enumerated in the agreement, or if the EUA is revoked and the
federal government determines after a reasonable time that it
is not likely to be restored, the government can terminate the
agreement. A no-cost settlement to end Pfizer’s performance
is expected within 30 days of the government providing notice.
Moderna
August 2020
$1.2 billion
100 million doses
Payment for meeting incremental
milestones without EUA required.
b
If Moderna fails to obtain an EUA, the federal government can
terminate the contract. The government can also terminate the
contract for convenience otherwise, in accordance with FAR §
52.249-2. The government reduced the stop-work period to no
more than 30 days, less than the usual 90-day period under
Federal Acquisition Regulation (FAR) § 52.242-15, in
consideration of the expedited award and performance under
the contract. In the event that the government issues a stop-
work order and Moderna continues to work, Moderna shall not
be entitled to an equitable adjustment by the government.
Janssen
August 2020
$1 billion
100 million doses
Payment for meeting incremental
milestones without EUA required.
If Janssen ceases to develop the vaccine for reasons
enumerated in the agreement, or if the EUA is revoked and the
federal government determines after a reasonable time that it
is not likely to be restored, the government can terminate the
agreement. Janssen would be entitled to full payment for
regimens for which manufacturing had been completed, but
which had not yet been delivered. With respect to regimens for
which manufacturing had been initiated but not completed,
Janssen would be entitled to payment of a proportion of the
price based on percentage of the work performed, among
other things.
If Janssen fails to comply with current good manufacturing
practices and that failure results in a material adverse effect,
Janssen will have 30 days to cure the failure. If Janssen fails to
take appropriate action the government can terminate the
contract.

Page 20 GAO-21-443 COVID-19 Vaccines
Company/initial award
date/value/quantity Payment Termination
AstraZeneca
October 2020
$1.2 billion
100 million doses
Payment for meeting incremental
milestones without EUA required.
c
The federal government can terminate its agreement with
AstraZeneca for certain event-based reasons, including the
Food and Drug Administration (FDA) placing clinical trials on
hold by FDA for a certain period of time, FDA revoking an
existing EUA, or AstraZeneca discontinuing development for
safety or efficacy reasons. The government can also terminate
its agreement if DOD decides termination is in its best
interests.
In the event the government terminates the contract for event-
based reasons, AstraZeneca is entitled to payment based on a
percentage of work completed and reasonable charges that
AstraZeneca can demonstrate resulted from the termination. In
addition to the foregoing, in the event of a termination in the
government’s best interest, AstraZeneca will be paid in full for
certain milestones regardless of percentage of work
completed.
Novavax
July 2020
$1.6 billion
100 million doses
Payment for meeting incremental
milestones without EUA required.
The government may terminate work under the agreement if
the government determines that a termination is in its interest.
The government and Novavax will negotiate equitable
reimbursement for work performed toward accomplishment of
the task or tasks of individual projects.
Sanofi
July 2020
$1.8 billion
100 million doses
Payment for meeting incremental
milestones without EUA required.
The government may terminate work under the agreement if
the government determines that a termination is in its interest.
The government and Sanofi will negotiate equitable
reimbursement for work performed toward accomplishment of
the task or tasks of individual projects.
Source: GAO analysis of Federal Procurement Data System – Next Generation data, award and other acquisition related documents and information provided by Operation Warp Speed officials. |
GAO-21-443
a
The federal government did not provide funding for development of the Pfizer vaccine; instead, it
purchased doses. Subsequent to this Department of Defense (DOD) other transaction agreement,
DOD awarded Pfizer a separate contract for 100 million doses in December 2020. DOD exercised a
contract option for 100 million additional doses in February 2021.
b
DOD exercised options under Moderna’s contract in December 2020 for 100 million doses and in
February 2021 for an additional 100 million doses.
c
Subsequent to this DOD other transaction agreement, in October 2020, DOD awarded AstraZeneca
a contract for 200 million additional doses.
According to DOD and HHS officials, the intellectual property underlying
the vaccine technology for the six vaccine candidates—including
intellectual property necessary for the formula and know-how to produce
it—was developed by the vaccine companies, or, in part, by the
government, before any funding was provided under Operation Warp
Speed and the continued DOD and HHS partnership. As a result, the
government does not have rights to the majority of intellectual property
developed prior to Operation Warp Speed-related efforts, according to
these officials.
Federal Government
Rights to Intellectual
Property Generally Limited
to Technical Data

Page 21 GAO-21-443 COVID-19 Vaccines
DOD and HHS contracting officials stated that they evaluated the
preexisting intellectual property to ensure that the companies had the
legal rights either through ownership or license to the patents that were
needed to enter into an agreement with the government to develop or
produce vaccines. According to DOD officials, this evaluation was also
done to determine whether any previous federal government investment
existed, and if so, to determine the scope of any previous investment and
rights and to inform each party’s negotiating position. For example, these
officials said that during the contract award process a DOD legal team
worked with HHS and Defense Advanced Research Projects Agency
attorneys to discuss related prior work to form a basis for the
negotiations.
36
Officials said DOD concluded that the government did not
own, in whole or in substantial part, the underlying intellectual property or
associated patents that the six companies identified as essential to
developing or manufacturing their specific vaccine candidates. For
example, DOD’s contract with Moderna includes an attachment
identifying Moderna’s background intellectual property. Moderna has also
publicly identified seven issued U.S. patents relevant to its vaccine, none
of which listed government inventors. At the same time, government
officials and company representatives noted that government scientists
had contributed to Moderna’s vaccine development and that some
government co-owned intellectual property could result from those
contributions.
DOD officials said in the absence of full rights to the underlying
intellectual property, and as part of DOD’s strategy to ensure the
availability of vaccines, they developed a unique contract clause to
address a potential scenario in which a vaccine company cannot ensure
36
Generally, the government only has rights in a company’s preexisting or “background”
intellectual property if there has been some previous agreement with the company. We
have found past instances in which the government and vaccine companies entered into
agreements to develop vaccine technology, but we do not have enough information about
the technology that was developed under those agreements or the nature of the rights that
were negotiated. For example, DOD’s price and cost analysis for the Moderna vaccine
noted that the Defense Advanced Research Project Agency awarded Moderna an up-to-5-
year, $24.6 million grant in October 2013 to research and develop its mRNA platform.
However, DOD’s price analysis did not provide additional information on this grant or
resulting patent information. DOD officials said DOD conducted a separate assessment of
the government’s underlying interest or co-ownership of background intellectual property
used in producing the new COVID-19 vaccine. We did not independently assess or
validate DOD’s process to review the company’s background intellectual property
assertions nor did we review the specific terms of the company’s previous government-
funded efforts.

Page 22 GAO-21-443 COVID-19 Vaccines
sufficient supply of doses subject to the terms of the contract or
agreement, and makes a business decision to stop production or sale of
the vaccine, or files for bankruptcy, among other things. For these
situations, DOD worked with companies to negotiate additional
government rights requiring the company to license the intellectual
property necessary to pursue FDA authorization or licensure, if required,
and manufacturing of the vaccine so that a third party may produce the
vaccine for exclusive sale to the government.
DOD officials said the intellectual property licensed would include the
vaccine formula and the technical know-how to produce it.
37
DOD officials
indicated, however, that implementing this approach would have some
practical limitations and considerations, such as whether the costs and
time associated with transferring technology to a third party might
outweigh the benefits. For example, the government or third party would
need to have the expertise and facilities necessary to use the licensed
intellectual property. These officials said they would need to determine
whether it would instead be more prudent to buy additional vaccine doses
from another company.
The additional rights DOD negotiated to allow for third-party
manufacturing of a vaccine do not apply in other potential scenarios,
which include:
• if a vaccine company experiences quality control or manufacturing
problems under its existing contract but does not make a business
decision to stop production or sale of the vaccine,
• if the government wants to increase the availability of a particular
vaccine by authorizing a different vaccine company to manufacture it,
or
• if a company significantly increases its proposed vaccine price on
subsequent contracts (i.e., an attempt to “price gouge”).
37
This language was included in five of the six companies’ contracts or agreements,
excluding Pfizer. According to DOD officials, DOD was unable to negotiate with Pfizer to
include third party manufacture as a remedy in its agreement due to the government’s lack
of involvement in the Pfizer vaccine’s development. Pfizer officials noted that Pfizer’s
agreement does not allow the government to “march-in,” as that term is defined in 35
U.S.C. § 203, and according to their agreement, government funding was limited to
payment for doses.

Page 23 GAO-21-443 COVID-19 Vaccines
While these other potential scenarios could create similar risks and
challenges, DOD officials identified mitigating circumstances or strategies
that minimized the risk. For example, DOD officials told us that the
expected availability of multiple vaccines would promote competition
between multiple vaccine companies and would make any price gouging
unlikely. Further, DOD officials stated that should the government need to
increase domestic manufacturing capacity to make certain vaccines, it
could invoke the Defense Production Act.
38
For example, the government
could modify existing priority ratings for specific companies to allow them
to obtain materials more quickly or they could expand manufacturing
capacity for supplies such as syringes.
39
DOD and HHS contracting officials noted that they are not aware of any
new intellectual property that will be created in the form of a “subject
invention” as a result of U.S. government funding for the Operation Warp
Speed-related contracts and agreements.
40
However, DOD generally
negotiated rights to the technical data—typically the scientific or
engineering data—generated under the production contract or
agreements, as applicable.
According to DOD contracting officials, the data the government may
obtain under the contracts and other agreements is generally limited to
technical data derived from the manufacturing scale-up process, such as
batch records and quality-related data. Generally, officials said, this
technical data gives the government insights into the vaccine production
process. In addition, having government representatives on site at
manufacturing plants allows them to observe the production process and
38
The Defense Production Act, as delegated, generally provides federal agencies
authority to, among other things, place priority ratings on contracts so that they receive
priority treatment over any other unrated contracts or orders if necessary to meet the
delivery or performance dates specified in the order; and provide financial incentives to
help maintain, restore, or expand the domestic industrial base. See Pub. L. No. 81-774, 64
Stat. 798 (1950) (codified, as amended, at 50 U.S.C. § 4501, et seq.); Exec. Order No.
13,603, 77 Fed. Reg. 16,651 (Mar. 22, 2012); 15 C.F.R. Part 700, Sch. 1 (2020).
39
DOD and HHS partnership officials stated that all six vaccine candidates have priority
ratings under the Defense Production Act.
40
The term “subject invention” means “any invention of a contractor conceived or first
actually reduced to practice in the performance of work under a funding agreement.” 35
U.S.C. § 201(e).

Page 24 GAO-21-443 COVID-19 Vaccines
any challenges that arise.
41
The related technical data the companies
provide allows for government oversight of production activities and
regulatory compliance according to DOD officials, but not access to the
vaccine formulas or know-how required to produce them.
The DOD and HHS partnership has taken several actions to increase the
availability of COVID-19 vaccine doses. As of March 25, 2021,
partnership officials stated that their aim is to have enough vaccine doses
distributed for all adults in the U.S.—about 265 million people—by the
end of May 2021.
42
They told us they expect to meet this time frame given
their additional purchase of vaccine doses and manufacturing expansion
efforts, which will lead to increased distribution of vaccines to jurisdictions
and federal partners, as described below.
Purchasing vaccine doses. As of April 1, 2021, DOD and HHS have
announced the purchase of at least 1.2 billion COVID-19 vaccine doses,
including 200 million doses each from Pfizer and Moderna, to be
manufactured and released by May 31, 2021 (see table 4). This is a
significant increase from the total of 600 million vaccine doses the federal
government had contracted to purchase as of November 2020.
Table 4: Contracted Amount of COVID-19 Vaccine Doses under the Department of Defense (DOD) and Department of Health
and Human Services (HHS) Partnership, by Vaccine Company, as of April 1, 2021
Vaccine company
Contracted amount
(millions of doses)
Contracted amount expected by the
end of May 2021 (millions of doses)
Emergency use
authorization (EUA)
Pfizer
300
a
200
b
Moderna
300
c
200
Janssen
100
87
AstraZeneca
300
d
TBD
e
Novavax
100
f
TBD
g
Sanofi
100
TBD
h
Total
1,200
487
Source: GAO analysis of award and other acquisition related documents and information from HHS, DOD, Advanced Technology International, and vaccine companies. | GAO-21-443
41
According to Pfizer representatives, since Pfizer did not accept U.S. government funding
under its agreement, the agreement did not include a “person in plant” provision to allow a
federal government official to observe its vaccine production process.
42
FDA authorized Pfizer’s vaccine for individuals 16 years of age and older, and
authorized Moderna’s vaccine for individuals 18 years of age and older. DOD and HHS
partnership officials told us that some vaccine companies have begun clinical trials for
adolescent and pediatric populations. On March 2, 2020, the White House announced
there would be sufficient doses for all adults in the U.S. by the end of May 2021.
The Federal
Government Has
Purchased Additional
Vaccine Doses and
Helped Mitigate
Manufacturing
Challenges to
Increase Vaccine
Availability

Page 25 GAO-21-443 COVID-19 Vaccines
Note: The contracted amount includes base and exercised options.
a
The federal government first awarded Pfizer an other transaction agreement for 100 million vaccine
doses. On December 22, 2020, the federal government awarded Pfizer a contract for an additional
100 million doses. On February 11, 2021, the government exercised another option in the Pfizer
contract for an additional 100 million doses, for a total of 300 million doses to be delivered by the end
of July 2021.
b
According to a company representative, Pfizer remains on track to fulfill its commitment to the federal
government to reach the 200 million doses mark by the end of May, and 300 million total doses by the
end of July.
c
The federal government first contracted with Moderna for 100 million vaccine doses. On December
11, 2020, the federal government announced that it had exercised an option with Moderna for an
additional 100 million doses. This was followed by an announcement on February 11, 2021, that the
government had exercised another option with Moderna for an additional 100 million doses, for a total
of 300 million doses.
d
The federal government awarded AstraZeneca an other transaction agreement for 100 million doses,
and later awarded AstraZeneca a contract for an additional 200 million doses, for a total of 300 million
doses, which will be provided to the government on a rolling basis, according to the company.
e
According to an AstraZeneca representative, the federal government and AstraZeneca are working
closely on a delivery schedule for the doses.
f
According to a Novavax representative, the company was also awarded a DOD contract in June
2020 that includes the delivery of 10 million doses. We do not include that amount in this table.
g
According to a Novavax representative, delivery of doses is dependent upon an EUA being granted
by the Food and Drug Administration.
h
According to a Sanofi representative, the release of the company’s vaccine doses is dependent upon
results of the phase 3 clinical study evaluating safety and efficacy, which is expected to begin in the
second quarter of 2021.
The 400 million purchased doses of the Pfizer and Moderna vaccines
expected by May 31, 2021 would be enough to fully vaccinate—that is to
provide 2 doses for each person being vaccinated, as necessary for each
of these vaccines—about 200 million people. In addition, Janssen is
expected to provide 87 million doses of its 1-dose vaccine by the end of
May 2021, which would be enough to vaccinate an additional 87 million
people. Combined with the expected 400 million doses from Moderna and
Pfizer (enough for 200 million people), the government would have
enough doses to vaccinate 287 million people which would exceed the
DOD and HHS partnership’s aim to have enough vaccine doses available
for all adults in the U.S. by the end of May 2021. Federal officials also
said that expected manufacturing of the AstraZeneca and Novavax
vaccine candidates would add to this total if those vaccine companies
request and receive EUAs to allow those vaccines to be marketed in the
United States.
Manufacturing and releasing vaccine doses. As of March 29, 2021,
226 million vaccine doses of the Pfizer, Moderna, and Janssen vaccines
had been manufactured and released, according to officials from the DOD

Page 26 GAO-21-443 COVID-19 Vaccines
and HHS partnership.
43
The number of vaccine doses released increased
notably at the end of February (see figure 4). According to information
from partnership officials, between December and mid-February of 2021,
an average of 8 million doses of the Pfizer and Moderna vaccines were
released per week. Between February 22, 2021, and March 29, 2021, this
increased to an average of 24 million doses per week. Representatives
from Pfizer and Moderna publicly stated that their vaccine manufacturing
rates would increase to 13 million doses per week for Pfizer by mid-March
and over 40 million doses per month—over 9 million doses per week—for
Moderna by April.
Figure 4: Number of Doses of COVID-19 Vaccine Released in the U.S. per Week (in millions), as of March 29, 2021
43
According to the DOD and HHS partnership, once through manufacturing and quality
assurance, the vaccine doses are released for distribution.

Page 27 GAO-21-443 COVID-19 Vaccines
Note: Numbers presented here are doses that have been released—that is, they have passed the
quality assurance process after manufacturing and been released for distribution, according to
officials from partnership between the Department of Defense (DOD) and Department of Health and
Human Services (HHS), formerly known as Operation Warp Speed.
DOD and HHS partnership officials said that they expect the rate of
vaccine doses manufactured and released to continue to increase. DOD
and HHS partnership officials and vaccine company representatives
described ways in which they continue to work together to mitigate
manufacturing challenges in order to increase manufacturing rates.
Specifically:
• Manufacturing capacity. Some vaccine companies have sought to
expand their capacity through making additional manufacturing sites
operational or modifying existing production facilities to manufacture
enough doses to fulfill their contracts with the federal government.
DOD and HHS partnership officials told us that they were working to
ensure that bottlenecks do not occur by helping companies obtain
additional fill-finish capacity for vaccines, with priority going to
vaccines with EUAs. For example, officials stated that they were
working with Catalent to add an additional production line at
Catalent’s fill-finish facility, which handles fill-finish manufacturing for
the Moderna and Janssen vaccines. Pfizer is also adding two
production lines at one of its fill-finish facilities. According to DOD
officials, the U.S. Army Corps of Engineers supported the fill-finish
facility upgrades by working with contractors to provide an additional
layer of quality control on behalf of the U.S. government. Officials
further stated that the U.S. Army Corps of Engineers leverages its
relationships with state and local governments in support of
manufacturers obtaining their permits and working with the
manufacturer’s suppliers in expediting the shipment of equipment and
supplies. Table 5 summarizes the status of the manufacturing
capacity expansion efforts for the six vaccine candidates under the
DOD and HHS partnership.
• Supply chains. Most vaccine company representatives indicated that
supply constraints were not impeding their manufacturing process as
of March 2021, and the companies have continued to utilize prioritized
contracts under the Defense Production Act to expedite the receipt of
supply constrained materials. These companies generally reported
that the priority rating process had been beneficial in providing
uninterrupted supply of materials. However, Janssen representatives
stated that providing priority ratings for multiple companies producing
COVID-19 vaccines may have unintended negative consequences,
such as creating constrained supplies for other life-saving medicines

Page 28 GAO-21-443 COVID-19 Vaccines
and unnecessary stockpiling of supplies that may be at risk of being
prioritized under the Defense Production Act. DOD and HHS
partnership officials also provided information that priority ratings for
COVID-19 vaccine materials are beginning to crowd out producers of
non-COVID-related life-saving therapies and medicines, including the
seasonal flu vaccine.
According to HHS, the administration is aware and working through
wider supply-chain disruptions resulting from the U.S. government use
of the Defense Production Act. HHS officials stated that it is important
to note that the supply-chain constraints are a result of unprecedented
global demand as global manufacturing races to produce vaccine for
more than 7 billion people using existing manufacturing infrastructure.
They further stated that the U.S. government’s use of the Defense
Production Act allows for prioritization of U.S. government contracts
for U.S.-based manufacturing, but it is not the primary cause of
shortages.
Additionally, partnership officials provided information on efforts to
mitigate risks from supply constrained materials. For example, officials
identified the potential risk of limited production capability for at least
one raw material for which supply has been hindered by (1) a fire at a
domestic facility which is delaying delivery of the material from this
facility by 4 to 10 weeks, and (2) an explosion at a foreign facility that
has required production to be shifted to a facility in a different country.
According to information provided by partnership officials, they are
closely monitoring shipments of this material from remaining foreign
sources and believe that this supply issue is unlikely to impact near-
term production due to supplies vaccine companies already have.
However, these supply disruptions highlight the overall fragility of the
global supply chain and the risks of relying on raw materials from a
small number of primarily foreign sources.
• Available workforce. The six vaccine companies indicated that
workforce gaps were not impeding their manufacturing process as of
March 2021. However, there continues to be a need to recruit and
train the highly skilled workforce needed to run vaccine production
processes.
44
As of March 2021, DOD personnel were continuing to
serve as quality control staff at the Emergent BioSolutions
manufacturing partner site until the company could hire the required
44
For example, Janssen representatives noted that each of the company’s manufacturing
partner sites continues to have vacancies, with sites that are still ramping up production
having a greater numbers of vacancies. Pfizer representatives stated that hiring staff with
specialized skills is always a challenge, but in general the company has been successful
in hiring qualified people.

Page 29 GAO-21-443 COVID-19 Vaccines
personnel, according to partnership officials. Although the DOD
personnel were originally scheduled to depart in mid-February 2021,
partnership officials told us that DOD personnel were extended to stay
until mid-April as Emergent BioSolutions continued to recruit, hire, and
train additional staff.
Table 5: Status of Manufacturing Capacity Expansion Efforts for Six COVID-19
Vaccine Candidates under the DOD and HHS Partnership, as of March 2021
Vaccine
company
U.S. manufacturing capacity expansion efforts
Pfizer
Has made and continues to make improvements and expansion at its
current facilities
Moderna
Completed work on near-term planned capacity; contracted with an
additional fill-finish manufacturing partner; making new capital
investments to increase capacity at its owned and partnered
manufacturing facilities
Janssen
Continuing to add additional manufacturing capacity, including
contracting with an additional manufacturing partner
AstraZeneca
Operating at full capacity in the United States
Novavax
Continuing to explore options for additional manufacturing capacity
Sanofi
Large-scale manufacturing has been delayed due to the results of the
combined phase 1 and 2 clinical trial
Source: GAO analysis of information from vaccine companies. | GAO-21-443.
Note: The Department of Defense (DOD) and Department of Health and Human Services (HHS)
partnership was formerly known as Operation Warp Speed.
Distributing vaccine doses. As of March 27, 2021, the partnership had
distributed 180.6 million total doses, according to data from CDC. The
number of vaccine doses distributed to jurisdictions and federal partners
has varied each week since the process began in December 2020. From
February 28, 2021 to March 27, 2021, the partnership distributed an
average of about 21.1 million doses of the Pfizer, Moderna, and Janssen
vaccines per week (see figure 5).
45
Because the Pfizer and Moderna
vaccines are 2-dose vaccines and the Janssen vaccine is a 1-dose
vaccine, this average weekly number of doses from each manufacturer
45
We chose this 4-week period to include the initial release of the Janssen vaccine during
the week of February 28, 2021, plus 3 additional weeks to reflect week-to-week variation.
The 21.1 million average number of doses per week included about 11.0 million doses of
Pfizer, 8.9 million doses of Moderna, and 1.2 million doses of Janssen vaccines (when
rounding).

Page 30 GAO-21-443 COVID-19 Vaccines
corresponds to enough doses each week for about 11.2 million people to
eventually be fully vaccinated.
Figure 5: Number of Vaccine Doses Distributed in the U.S. per Week, as of March
27, 2021
Note: Vaccine doses distributed is the total number that have been delivered to jurisdictions, retail
pharmacies, long-term care facilities, Federal Emergency Management Agency and Health
Resources and Services Administration partner sites, and federal entities. However, for Palau,
Micronesia, Marshall Islands, Guam, American Samoa, and Northern Marianas Islands, total counts
of COVID-19 vaccine doses include doses marked as shipped in CDC’s Vaccine Tracking System
since December 13, 2020. For COVID-19 vaccination there are 64 jurisdictions including all 50 states,
territories, and local health programs in Chicago, the District of Columbia, Houston, New York City,
Philadelphia, and San Antonio.
a
For the week ending on February 27, 2021, the national doses distributed totals show larger than
typical daily increases. This is an accurate reflection of the data and is the result of weather events
causing a backlog of vaccine distributed to many parts of the United States.
DOD and HHS partnership officials noted that projecting future vaccine
dose availability beyond approximately 3 weeks is inherently uncertain, in
part because of the complex manufacturing processes for biologics like

Page 31 GAO-21-443 COVID-19 Vaccines
vaccines, as well as the manufacturing challenges discussed earlier.
46
Further, while they indicated that they expect the number of vaccine
doses distributed to increase each week in line with the expected
increases in vaccine manufacturing, there may be slight differences due
to the time it takes to package and ship the doses.
With the expected increases in vaccine manufacturing and distribution,
HHS announced on March 17, 2021 that the Acting Secretary of Health
and Human Services was directing all states, Tribes, and territories to
make all adults in the U.S. eligible for the vaccine by May 1, 2021.
47
Effective communication on vaccine availability will be critical to manage
public expectations, particularly given the uncertainty with future
projections and the significant effort required to manufacture and
distribute sufficient vaccine doses available for all adults in the U.S. In
particular, having sufficient vaccine doses available by the end of May
2021 will generally require an increase of more than half of weekly
distribution amounts compared to the average weekly rate for February
28 through March 27, 2021.
48
In our prior work on the H1N1 influenza pandemic, we found that although
the federal government was able to purchase and distribute millions of
doses of H1N1 vaccine, the vaccine was not widely available when the
public expected it, and the failure to effectively manage public
expectations undermined government credibility.
49
As we reported in
January 2021, initial vaccine rollout did not match public expectations,
with initial numbers of distributed and administered COVID-19 vaccines
46
The manufacturing of vaccines can be more uncertain than drug-related manufacturing,
in part because vaccines often need to grow living material that can take time and be
imprecise. DOD and HHS partnership officials told us that projecting future vaccine doses
availability was also difficult because of the inherent challenge with scale-up and
production in a short timeframe.
47
On April 6, 2021, the President revised this, announcing that all states, Tribes, and
territories would be directed to make all adults in the U.S. eligible by April 19, 2021.
48
The average weekly rate from February 28 through March 27 corresponds to enough
doses each week for about 11.2 million people to eventually be fully vaccinated, based on
the number of 2-dose and 1-dose vaccines distributed during this time. We calculated that
this weekly rate would need to increase by about 59 percent, on average, to have enough
doses available by May 31 to eventually allow for all adults in the U.S. to be fully
vaccinated. Our calculation assumes that the proportion of 2-dose and 1-dose vaccines
would remain the same.
49
See GAO, Influenza Pandemic: Lessons from the H1N1 Pandemic Should Be
Incorporated into Future Planning, GAO-11-632 (Washington, D.C., Jun. 27, 2011).

Page 32 GAO-21-443 COVID-19 Vaccines
falling short of implementation expectations set by officials.
50
Further, as
we have reported since June 2020, timely, clear, and consistent
communication is essential to ensure public confidence and trust.
Stakeholders—including representatives of state, territorial, and local
health officials and health care providers—identified multiple challenges
with initial implementation of COVID-19 vaccination, which includes
prioritizing, allocating, distributing, and administering available vaccine
doses to individuals. Through our interviews with stakeholders conducted
from January 6 through February 1, 2021, when vaccine doses were
initially available, we found that state, territorial, and local health officials
and health care providers faced challenges such as competing priorities
with limited resources and lack of information needed for on-the-ground
planning for vaccine administration. Table 6 provides examples of
challenges in the initial COVID-19 vaccine implementation identified by
some of these stakeholders.
50
See GAO, COVID-19: Critical Vaccine Distribution, Supply Chain, Program Integrity, and
Other Challenges Require Focused Federal Attention, GAO-21-265 (Washington, D.C.:
Jan. 28, 2021).
Stakeholders
Identified Challenges
with Initial COVID-19
Vaccination; the
Federal Government
Has Taken Some
Steps to Help
Improve Vaccine
Implementation
Stakeholders Identified
Multiple Challenges with
Initial COVID-19
Vaccination
Implementation, Which
Were Exacerbated by
Changing Federal
Procedures

Page 33 GAO-21-443 COVID-19 Vaccines
Table 6: Examples of Challenges with Initial COVID-19 Vaccine Implementation Reported by Stakeholders, as of February 1,
2021
Challenge
Description
Health officials and health care
providers faced competing
priorities with limited resources
Some stakeholders, including representatives of state, territorial, and local health officials and
health care providers, said that they faced challenges in trying to plan for and conduct vaccine
distribution and administration activities while also managing their other COVID-19 response
efforts, such as contract tracing. Prior to December 14, 2020, when vaccination began, some
stakeholders indicated limited resources were available for planning these efforts. For example,
some stakeholders said they needed to hire and train new staff to enhance existing data systems to
handle the anticipated increase in data collection or to run vaccination clinics, while also treating
patients.
a
Health officials and health care
providers lacked information on
vaccine shipments needed for
on-the-ground planning
Stakeholders said some state, territorial, and local health officials and health care providers lacked
information on vaccine shipments, such as on the number of doses they would receive and when
they would arrive, which is critical to planning for and administering vaccinations. For example, one
stakeholder noted not having this information makes it difficult for states to plan further distribution
to the local level. Another stakeholder noted that this information is critical for planning
vaccinations, particularly for health care providers in rural communities that may not have hospitals.
These communities might use mobile vans or recreational centers that require advance planning so
that people can be at a particular location on a specific day.
Health officials identified
challenges with reporting
administration data to the
Centers for Disease Control and
Prevention (CDC)
Stakeholders said some state, territorial, and local health officials have experienced challenges
affecting their ability to report vaccine administration data to CDC. For example, health officials in
one state had to update their data reporting systems with new functionality for the pandemic, and
there have been some interoperability challenges with getting access to administration data. One
stakeholder also noted there can be as much as a 4-5 day delay in reporting data from the health
care provider to CDC because health care providers have up to 72 hours to report administration
data to the jurisdictions and jurisdictions have 24 hours to review these data before reporting them
to CDC.
Local health departments and
health care providers could not
plan for their vaccination efforts
because they were not included
in federal planning efforts, which
focused on coordination at the
jurisdictional (e.g., state) level
Some local health departments and some health care providers did not participate in planning
exercises organized by the federal government because federal planning efforts focused on
coordination at the jurisdictional (e.g., state) level, according to stakeholders. Thus, stakeholders
said some local health departments and some health care providers did not have key information
needed for planning, such as when and how many vaccine doses would be arriving. This lack of
information affected some local health departments’ and some health care providers’ ability to
coordinate and plan, including their ability to develop their own community education and
messaging around vaccination.
Local health departments and
health care providers were
uncertain on who to vaccinate or
where to provide vaccinations
Some local health departments were not initially informed when jurisdictions deviated from federally
recommended groups targeted for vaccination, resulting in uncertainty about who they should
vaccinate, according to one stakeholder representing local health officials. For example, when the
Secretary of Health and Human Services recommended expanding the initial vaccination target
group from persons aged 75 and older to those 65 and older, this stakeholder said there was little
or no planning done by local communities, including by local health departments and health care
providers, for such an expansion.
Additionally, some hospital officials did not initially know whether they were responsible for
vaccinating health care providers in their community who were eligible to be vaccinated but were
not affiliated with a particular hospital, according to one stakeholder. Similarly, unaffiliated providers
did not know where or when they could get vaccinated when they were in the initial priority group
for vaccination, according to another stakeholder.

Page 34 GAO-21-443 COVID-19 Vaccines
Challenge
Description
Health care providers did not
have information from an
authoritative source to combat
misinformation and vaccine
hesitancy
b
Some stakeholders said health care providers needed science-based information to combat
misinformation and vaccine hesitancy during the initial vaccine implementation. For example, one
stakeholder noted that certain spokespeople made inaccurate statements about who could be
vaccinated. Further, some heath care providers were hesitant to be vaccinated because of
misinformation about the vaccines, resulting in a lower proportion of health care staff in long-term
care facilities willing to be vaccinated than initially expected, according to some stakeholders.
Additionally, another stakeholder noted that health care providers of color were more likely to
refuse a COVID-19 vaccine when offered than their white counterparts, and a second stakeholder
said that some members of the public remain hesitant to be vaccinated, in part, due to
misinformation about the vaccines.
Source: GAO analysis of interviews with representatives of state, territorial, and local health officials, and health care providers from January 6, 2021, through February 1, 2021. | GAO-21-443
Note: These challenges were examples identified collectively by stakeholders and not by every
stakeholder interviewed.
a
Prior to the beginning of vaccine administration on December 14, 2020, approximately $340 million
had been made available from CDC to jurisdictions for vaccine preparedness. Of this $340 million,
the Department of Health and Human Services announced $200 million would be awarded on
September 23, 2020, and an additional $140 million would be awarded on December 15, 2020.
b
For the purposes of this report, vaccine hesitancy refers to a delay in acceptance of vaccines despite
availability. Those who are vaccine hesitant are a middle group along a continuum that ranges
between those who fully accept vaccines on one end and those who are strongly opposed to
vaccines on the other. An individual’s vaccine hesitancy can apply to all vaccines or to a specific
vaccine.
Some stakeholders indicated these challenges were exacerbated by
unanticipated changes from the federal government. For example, in
December 2020, ACIP recommended that, when initial vaccine supplies
were expected to be limited, vaccine doses should first be offered to
health care personnel and long-term care facility residents. Next, vaccine
doses should be offered to persons aged 75 and older and non–health
care, frontline essential workers. As more vaccine doses became
available, ACIP recommended vaccination be expanded to include
persons aged 65 and older.
51
However, during a press conference on
January 12, 2021, the Secretary of Health and Human Services
recommended that the target group be expanded to include those aged
51
ACIP recommendations for phased allocation provide guidance for federal, state, and
local jurisdictions while vaccine supply is limited. To inform policy options for ACIP, the
committee’s COVID-19 Vaccines Work Group, comprising experts in infectious diseases,
vaccinology, vaccine safety, public health, and ethics, held 28 meetings to review data
regarding vaccine candidates, COVID-19 surveillance, modeling of allocation scenarios,
and vaccination program implementation issues. In its deliberations, ACIP considered
scientific evidence regarding COVID-19 epidemiology, ethical principles, and vaccination
program implementation. ACIP recommendations are reviewed by the CDC Director and,
if adopted, are published as official CDC/HHS recommendations in the Morbidity and
Mortality Weekly Report, https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm695152e2-
H.pdf.

Page 35 GAO-21-443 COVID-19 Vaccines
65 and older and people under 65 with a documented co-morbidity before
additional vaccine doses became available.
At the same time, jurisdictions experienced an unexpected decline in their
second-week allocations of vaccine doses and some said they received
mixed messages from the federal government on vaccine availability. See
table 7 for changes made by the federal government regarding COVID-19
vaccinations that occurred during the initial vaccine implementation
through mid-February 2021. Some stakeholders expressed frustration
with these unanticipated changes and stressed the need for more
predictable information and guidance going forward, underscoring the
need for clear and consistent coordination and communication by the
federal government to help ensure more effective vaccine
implementation.
Table 7: Changes by the Federal Government to the Initial COVID-19 Vaccine Implementation between November 2020 and
mid-February 2021
Change
Date
Event
Expansion of
recommended target
groups to receive initial
supplies of vaccine doses
beyond initial
recommendations
Dec. 1-2, 2020
The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on
Immunization Practices (ACIP) recommended that when supplies of COVID-19
vaccines are limited, vaccination should be offered in a phased approach. ACIP
recommended that vaccines in the initial phase of the vaccination program (phase 1a)
be offered to (1) health care personnel, and (2) residents of long-term care facilities.
a
The CDC Director adopted ACIP’s recommendation for priority groups for the initial
phase of the COVID-19 vaccination program.
b
Dec. 20-21, 2020
ACIP recommended that after phase 1a, vaccination should be offered in phase 1b to:
(1) persons aged 75 and older and (2) frontline essential workers (non-healthcare);
and in phase 1c to: (1) persons aged 65-74 years, (2) persons aged 16-64 years with
high-risk medical conditions, and (3) other essential workers. The CDC Director
adopted ACIP’s updated recommendations for priority groups for allocation of COVID-
19 vaccine doses.
b
Jan. 12, 2021
The Secretary of Health and Human Services announced at a press briefing that
jurisdictions should open vaccination to all persons age 65 and older and all people
under age 65 with a documented co-morbidity.
Jan. 21, 2021
The White House released the National Strategy for the COVID-19 Response and
Pandemic Preparedness (National Strategy) that encouraged jurisdictions to open
vaccination to persons age 65 and older and essential workers.
c

Page 36 GAO-21-443 COVID-19 Vaccines
Change
Date
Event
Changes in number of
doses, including in states’
and other jurisdictions’
second week allocations
of the Pfizer and Moderna
vaccines
Late Dec. 2020
For 51 jurisdictions (93 percent of the 55 jurisdictions for which data were available),
the number of Pfizer vaccine doses included in their second week’s allocation was
more than 25 percent lower than were included in their first week’s allocation,
according to CDC data.
d
An Operation Warp Speed official explained that the number of doses available for
allocation in the second week of the Pfizer vaccine implementation was lower [than
initially understood] and so the actual allocations made were lower than had originally
been communicated to jurisdictions.
For 54 jurisdictions (98 percent of the 55 jurisdictions for which data were available),
the number of doses available for allocation in the second week of Moderna vaccine
implementation was about 65 percent lower than their first allocation.
e
Jan. 6, 2021
The Food and Drug Administration (FDA) determined six doses instead of five doses
can be obtained from the Pfizer vaccine vials by using “low dead-volume” syringes. If
standard syringes and needles are used, there may not be sufficient vaccine to extract
a sixth dose.
f
Changes in federal
management of vaccines
for second doses
Dec. 2, 2020
During a press conference, an Operation Warp Speed official said the federal
government would not distribute the second dose of vaccine to jurisdictions to ensure
that enough vaccine doses would be available for people to receive it. The official said
the federal government would distribute the second dose of the vaccine either 3 or 4
weeks later, depending on the vaccine.
g
Dec. 9, 2020
During a press conference, an Operation Warp Speed official said the federal
government set aside some vaccine doses from the initial total supply as part of a
reserve.
h
This official said that as the federal government became more confident in
the manufacturing and distribution process, a smaller reserve of vaccine doses would
be maintained.
Jan. 12, 2021
During a press conference, the Secretary of Health and Human Services said the
federal government was releasing its entire supply of vaccine for jurisdictions to order
rather than holding back second doses in a physical reserve until a 3- or 4-week time
period had passed. This statement led some jurisdictional officials (e.g., state officials)
to anticipate they would receive a larger weekly allocation of vaccine doses.
Jan. 15, 2021
In a press interview, the Secretary of Health and Human Services clarified that the
federal government did not have a stockpile of reserved second doses of vaccine.
Jan. 21, 2021
The National Strategy stated that the administration planned to end the policy of
holding back significant levels of vaccine doses. The National Strategy also noted that
to ensure the availability of second doses of vaccine, the administration would hold a
smaller reserve and closely monitor the development, production, and release of
vaccine doses, and use the Defense Production Act as needed to ensure adequate
supply.
Announced modifications
to how vaccines would be
allocated
Nov. 2020
In anticipation of the authorizations of the Pfizer and Moderna vaccines, Operation
Warp Speed officials reported that allocations would be made based on jurisdictions’
adult populations (i.e., those aged 18 years and older).

Page 37 GAO-21-443 COVID-19 Vaccines
Change
Date
Event
Jan. 12, 2021
In a press conference, the Secretary of Health and Human Services announced that
beginning in 2 weeks, vaccines would be allocated to jurisdictions based on the
percentage of doses each jurisdiction had administered along with the number of
residents aged 65 years and older residing in the jurisdiction.
Feb. 11, 2021
Department of Defense (DOD) and Department of Health and Human Services (HHS)
partnership officials said the allocation policy for jurisdictions would be based on
jurisdictions’ adult populations (i.e., those 18 and older).
Source: GAO analysis of CDC data on vaccine allocations, federal planning and other documents, transcripts of press conferences with Operation Warp Speed officials, which included HHS and DOD
officials, and interviews with federal officials. | GAO-21-443.
Note: COVID-19 vaccine implementation includes prioritization, allocation, distribution and
administration of any authorized or licensed COVID-19 vaccine. Also, as of January 2021, the
partnership between DOD and HHS has continued but is no longer referred to as Operation Warp
Speed.
a
CDC defines health care personnel as paid and unpaid persons serving in health care settings who
have the potential for direct or indirect exposure to patients or infectious materials. Long-term care
facility residents are defined as adults who reside in facilities that provide a range of services,
including medical and personal care, to persons who are unable to live independently. See
Department of Health and Human Services, Centers for Disease Control and Prevention, “The
Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial
Supplies of COVID-19 Vaccine—United States, 2020,” Morbidity and Mortality Weekly Report,
(Atlanta, Ga.: Dec. 11, 2020), accessed on March 1, 2021,
https://www.cdc.gov/mmwr/volumes/69/wr/mm6949e1.htm.
b
ACIP recommendations are reviewed by the CDC Director and, if adopted, are published as official
HHS/CDC recommendations in the Morbidity and Mortality Weekly Report. See
https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html (accessed March 1, 2021) for
ACIP’s current COVID-19 vaccine recommendations. For the purposes of ACIP’s recommendation,
non-healthcare frontline essential workers include firefighters, police officers, corrections officers,
food and agricultural workers, U.S. Postal Service workers, manufacturing workers, grocery story
workers, public transit workers, those who are in the education sector (teachers and support staff), as
well as daycare workers. According to CDC, about 49 million persons, including non-healthcare
frontline essential workers and individuals aged ≥75 years are recommended for vaccination in phase
1b, and an additional 129 million persons are recommended for vaccination in phase 1c (including
about 28 million individuals aged 65-74 years).
c
White House, National Strategy for the COVID-19 Response and Pandemic Preparedness
(Washington, D.C.: Jan. 21, 2021), accessed on January 21, 2021 at
https://www.whitehouse.gov/wp-content/uploads/2021/01/National-Strategy-for-the-COVID-19-Respo
nse-and-Pandemic-Preparedness.pdf.
d
For COVID-19 vaccine implementation, there are 64 jurisdictions including all 50 states, territories,
the District of Columbia, and local health programs in Chicago, the District of Columbia, Houston,
New York City, Philadelphia, and San Antonio that receive vaccine allocations from Operation Warp
Speed and the continued HHS and DOD partnership. According to CDC, the Marshall Islands,
Micronesia, and Palau did not receive doses of the Pfizer vaccine due to logistical considerations with
ultra-cold storage requirements. American Samoa, Guam, and the Mariana Islands received their first
and second doses simultaneously to optimize transportation logistics. Alaska was allocated vaccine
doses the first and fourth weeks, but not the second or third week of Pfizer vaccine implementation.
Houston and San Antonio had their allocations consolidated with Texas.
e
According to CDC, for the Moderna vaccine, American Samoa, Guam, and the Mariana Islands
received their first and second doses simultaneously to optimize transportation logistics. Alaska, the
Marshall Islands, Micronesia, and Palau were allocated vaccine doses the third week but not the
second week of Moderna vaccine implementation. Houston and San Antonio had their allocations
consolidated with Texas.
f
Department of Health and Human Services, Food and Drug Administration, “Fact Sheet for
Healthcare Providers Administering Vaccine (Vaccination Providers): Emergency Use Authorization

Page 38 GAO-21-443 COVID-19 Vaccines
(EUA) of the Pfizer-BioNTech COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19)”,
accessed January 11, 2021, https://www.fda.gov/media/144413/download.
g
The second dose of the Pfizer vaccine should be administered 3 weeks after the first dose, and the
second dose of the Moderna vaccine should be administered 1 month after the first dose, according
to FDA.
h
The federal government maintained this emergency reserve of vaccine doses based on
recommendations from the National Security Council, according to federal officials.
In January 2021, HHS announced that CDC would provide more than $3
billion in awards using funds provided under the Consolidated
Appropriations Act, 2021, to jurisdictions to support vaccination-related
activities.
52
In addition, the American Rescue Plan Act of 2021, enacted in
March 2021, provided additional resources for vaccination activities. For
example, that act includes $7.5 billion for CDC to carry out activities to
plan, prepare for, promote, distribute, administer, monitor, and track
COVID-19 vaccines.
53
With the release of the National Strategy, the
federal government identified several specific initiatives for vaccine
implementation, as part of the larger COVID-19 response.
54
Several
examples of federal initiatives include:
Additional funding and resources for vaccine distribution and
administration. In accordance with a January 21, 2021 Presidential
Memorandum, the Federal Emergency Management Agency (FEMA) will
reimburse states, territorial, local, and tribal governments for costs
associated with COVID-19 response, which includes vaccine distribution
and administration, through the Disaster Relief Fund, which had a
52
The $3 billion in awards will be made through the CDC Immunization and Vaccines for
Children cooperative agreement, and funds will be allocated by a population-based
formula.
53
Pub. L. No. 117-2, § 2301, 135 Stat. 4, 37-38.
54
White House, National Strategy for the COVID-19 Response and Pandemic
Preparedness (Washington, D.C.: Jan. 21, 2021), accessed on January 21, 2021
https://www.whitehouse.gov/wp-content/uploads/2021/01/National-Strategy-for-the-
COVID-19-Response-and-Pandemic-Preparedness.pdf.
The Federal Government
Has Taken Some Steps to
Help Improve COVID-19
Vaccine Implementation

Page 39 GAO-21-443 COVID-19 Vaccines
balance of about $61.4 billion, as of March 18, 2021, according to
FEMA.
55
On February 18, 2021, FEMA also reported it could support establishing
community vaccination centers and provide additional resources (e.g.,
clinical staff including nurses and emergency medical services personnel)
and additional ancillary supplies (e.g., gloves, masks, and syringes).
These community vaccination centers range from mobile sites that can
support the administration of 250 vaccine doses a day to those that can
support the administration of 6,000 vaccine doses a day.
56
According to
FEMA, as of March 26, 2021, the agency had partnered with California,
Florida, Georgia, Illinois, Michigan, New York, North Carolina, Ohio,
Pennsylvania, and Texas to establish community vaccination centers and
planned to partner with additional states in late-March and April 2021.
57
55
White House, Memorandum to Extend Federal Support to Governors’ Use of the
National Guard to Respond to COVID-19 and to Increase Reimbursement and Other
Assistance Provided to States, (Washington D.C.: Jan. 21, 2021), accessed on February
4, 2021, https://www.whitehouse.gov/briefing-room/presidential-
actions/2021/01/21/extend-federal-support-to-governors-use-of-national-guard-to-
respond-to-covid-19-and-to-increase-reimbursement-and-other-assistance-provided-to-
states/.
On February 2, 2021, the administration announced it would retroactively reimburse states
fully for FEMA-eligible services—including masks, gloves, emergency feeding actions,
sheltering at risk populations, and mobilization of the National Guard—back dated to the
beginning of the pandemic in January 2020 in the United States, according to FEMA.
White House, Memorandum on Maximizing Assistance from the Federal Emergency
Management Agency, (Washington D.C.: Feb 2, 2021), accessed on February 4, 2021,
https://www.whitehouse.gov/briefing-room/presidential-actions/2021/02/02/memorandum-
maximizing-assistance-from-the-federal-emergency-management-agency/.
According to FEMA, as of March 31, 2021, it had provided more than $4.49 billion to 42
states, the District of Columbia, five territories, and four Tribes for expenses related to
COVID-19 vaccination efforts.
56
Federal Emergency Management Agency, Community Vaccination Centers Playbook,
Version 4.0 (Washington, D.C.: Mar. 26 2021), accessed on March 30, 2021,
https://www.fema.gov/sites/default/files/documents/fema_community-vaccination-
centers_playbook_03-26-2021.pdf.
57
See FEMA’s website on its support of COVID-19 vaccination efforts, accessed on
February 26, 2021, https://www.fema.gov/disasters/coronavirus/vaccine-support.
According to FEMA, the sites are selected based on data analysis including the CDC’s
Social Vulnerability Index and other Census data as well as input from state and local
partners.

Page 40 GAO-21-443 COVID-19 Vaccines
The National Strategy also proposed augmenting the number of health
care providers available to administer vaccines by, for example, utilizing
the Army and Navy Medical Corps. According to FEMA, as of March 26,
2021, 6,838 federal personnel were deployed to 997 federally supported
vaccination sites across the country, including 2,905 DOD personnel and
2,108 FEMA and Department of Homeland Security personnel who were
directly supporting vaccination efforts. There were also an additional
2,800 personnel serving in non-clinical operational roles. In addition, as of
March 26, 2021, U.S. National Guard Bureau personnel were supporting
the COVID-19 response, including 1,916 vaccinators.
Earlier advance notice on vaccine allocations and increased
amounts of projected supply. On January 26, 2021, the White House
announced that the federal government would begin providing states,
Tribes, and territories with reliable vaccine allocation estimates 3 weeks
in advance—instead of the one-week look-ahead they had been
receiving—to give state and local leaders greater certainty around supply
so they can plan their vaccination efforts.
58
In addition, on March 9, 2021,
the White House announced that it would increase the overall weekly
vaccine supply to states, Tribes, territories, and others to more than 20
million doses. This amount was up from an earlier weekly vaccine supply
of 8.5 million doses.
Initiation of a federal retail pharmacy program. In January 2021, CDC
announced a federal partnership with retail pharmacies, including 21
national pharmacy partners and networks of independent pharmacies,
representing over 40,000 pharmacy locations nationwide. Described in
CDC’s COVID-19 Vaccination Program Interim Playbook for Jurisdictions
Operations Annex (annex), CDC noted that not all pharmacy locations
would receive vaccine doses in the initial stages of implementation, but
58
White House, Fact Sheet: President Biden Announces New Steps to Boost Vaccine
Supply and Increase Transparency for States, Tribe, and Territories (Washington, D.C.:
Jan. 26, 2021), accessed on February 4, 2021, https://www.whitehouse.gov/briefing-
room/statements-releases/2021/01/26/fact-sheet-president-biden-announces-new-steps-
to-boost-vaccine-supply-and-increase-transparency-for-states-tribes-and-territories/.

Page 41 GAO-21-443 COVID-19 Vaccines
instead, jurisdictions were to select one or more pharmacy partners,
based on a pre-selected list of partners.
59
According to CDC, the pre-selected pharmacy partners were chosen
based on CDC analysis that they could provide vaccine to those in
greatest need, including those at highest risk of becoming severely ill
from COVID-19 and those who are socially vulnerable. According to the
annex, as the vaccine supply increases, the number of participating retail
pharmacies will also increase. Selected pharmacies received their initial
vaccine doses, which were separate from vaccine allocations provided to
jurisdictions, on February 11, 2021, according to the White House. On
February 16, 2021, the White House announced that 2 million doses
would be going to pharmacies that week.
Initiation of a federal partnership with federally qualified health
centers. The National Strategy described a federal partnership with
federally qualified health centers, and during the week of February 22,
2021, federally qualified health centers began directly receiving vaccine
allocations from the federal government.
60
Similar to the federal
partnership with retail pharmacies, this program began with a phased-in
approach, initially including 25 sites. The health centers initially chosen
include those that serve a large volume of disproportionately affected
populations, including individuals experiencing homelessness, migrant or
seasonal agricultural workers, and those with limited English proficiency,
according to the Health Resources and Services Administration.
61
Across
this initial phase, the goal is to allocate 1 million doses, according to a
White House COVID-19 Response Team statement on February 9,
59
Department of Health and Human Services, Centers for Disease Control and
Prevention, COVID-19 Vaccination Program Interim Playbook for Jurisdictions Operations
Annex (Atlanta, Ga.: Jan. 11, 2021), accessed on February 4, 2021,
https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-vaccination-program-
playbook-annex.pdf.
According to CDC, pharmacies will be administering COVID-19 vaccine in accordance
with the prioritization groups determined by the appropriate public health authority (e.g.,
the state or local health department in coordination with the state governor).
60
These allocations to federally qualified health centers are separate from the allocations
provided to jurisdictions.
61
See the Health Resources and Services Administration’s Ensuring Equity in COVID-19
Vaccine Distribution—Engaging Federally Qualified Health Centers website, accessed on
February 26, 2021, https://www.hrsa.gov/coronavirus/health-center-program.

Page 42 GAO-21-443 COVID-19 Vaccines
2021.
62
As of March 8, 2021, 199 health centers were participating and 51
additional health centers were invited to participate in this program,
according to the Health Resources and Services Administration.
Enhancing the number of eligible vaccinators and expanding
eligibility for vaccination to all adults in the U.S. by April 19, 2021.
On March 11, 2021, the administration announced plans to allow other
qualified health care providers to administer COVID-19 vaccine, including
dentists, midwives, optometrists, paramedics, veterinarians, and medical
and nursing students.
63
The White House also announced expectations
that all eligibility restrictions would be lifted by May 1, 2021, making all
adults in the U.S. eligible for vaccination. On April 6, 2021, the White
House announced it was moving that date up, from May 1, 2021, to April
19, 2021. The White House also expects to launch various tools to make
it easier for individuals to identify where they can be vaccinated. These
federally supported tools include a “Find a Vaccination Website” and a 1-
800 number for a call center that will provide guidance and assistance to
those who may lack internet access. In addition, the administration stated
it would deploy technology teams to states who need assistance in
improving the websites their states’ are using to schedule vaccination
appointments.
Information published on vaccine safety. In January and February
2021, CDC published results from its analysis of COVID-19 vaccine
safety monitoring, including allergic reactions reported after receipt of the
Pfizer and Moderna vaccines in its Morbidity and Mortality Weekly Report
and in medical articles, including data on reported cases of anaphylaxis,
62
See https://www.whitehouse.gov/briefing-room/press-briefings/2021/02/09/press-
briefing-by-white-house-covid-19-response-team-and-public-health-officials-2/, accessed
on February 26, 2021.
63
White House, Fact Sheet: President Biden to Announce All Americans to be Eligible for
Vaccinations by May 1, Puts the Nation on a Path to Get Closer to Normal by July 4th.
(Washington, D.C.: Mar. 11, 2021), accessed on March 15, 2021,
https://www.whitehouse.gov/briefing-room/statements-releases/2021/03/11/fact-sheet-
president-biden-to-announce-all-americans-to-be-eligible-for-vaccinations-by-may-1-puts-
the-nation-on-a-path-to-get-closer-to-normal-by-july-4th/.

Page 43 GAO-21-443 COVID-19 Vaccines
frequently reported adverse events, and deaths.
64
CDC also posted a
series of communication resources on its website, including tool kits for
health care providers, long term care facilities, employers of essential
workers, and community-based organizations that provide educational
information about the benefits of COVID-19 vaccination and address
common questions and concerns.
65
Additional guidance on equitable access promoting vaccination.
CDC’s January 2021 annex provided tools to jurisdictions on how to
increase vaccine confidence in priority populations. The document, which
focused, in part, on balancing equitable access with demand for vaccine,
included new guidance to jurisdictions on when and how to transition from
initial target populations to other priority populations and how to promote
increased vaccination.
These and other steps the federal government has initiated may take time
to implement at the federal, state, and local levels. Thus, it is too soon to
determine to what extent, these and other efforts outlined in the National
Strategy might help facilitate vaccine distribution and administration going
forward. As we have reported since June 2020, coordination and clear
and consistent communication across all levels of government and with
all stakeholders remains a critical component of a successful response to
the COVID-19 pandemic.
64
See, for example Department of Health and Human Services, Centers for Disease
Control and Prevention, “First Month of COVID-19 Vaccine Safety Monitoring—United
States, December 14, 2020—January 13, 2021, Morbidity and Mortality Weekly Report
(Atlanta, Ga.: Feb. 19, 2021), accessed February 26, 2021,
https://www.cdc.gov/mmwr/volumes/70/wr/mm7008e3.htm; “Allergic Reactions Including
Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine—United
States, December 21, 2020—January 10, 2021, Morbidity and Mortality Weekly Report
(Atlanta, Ga.: Jan. 22, 2021), accessed on February 26, 2021,
https://www.cdc.gov/mmwr/volumes/70/wr/mm7004e1.htm; “Allergic Reactions Including
Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—
United States, December 14-23, 2020, Morbidity and Mortality Weekly Report (Atlanta,
Ga.: Jan. 6, 2021), accessed on February 11, 2021,
https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm?s_cid=mm7002e1_w%E2%80
%8B; and T.T. Shimabukuro, M. Cole, J. R. Su, “Reports of Anaphylaxis After Receipt of
mRNA COVID-19 Vaccines in the US—December 14, 2020-January 18, 2021” JAMA. Vol.
325, no. 11 (2021): pp. 1101–1102.
https://jamanetwork.com/journals/jama/fullarticle/2776557?resultClick=1 (accessed April 5,
2021).
65
See https://www.cdc.gov/coronavirus/2019-ncov/vaccines/toolkits.html, accessed
February 26, 2021.

Page 44 GAO-21-443 COVID-19 Vaccines
Finally, the Consolidated Appropriations Act, 2021, required the CDC
Director to provide Congress with an updated and comprehensive
COVID-19 vaccine distribution strategy within 30 days of the law’s
enactment (by January 26, 2021).
66
In March 2021, CDC prepared an
initial submission of the strategy that provides a high-level description of
the agency’s ongoing and planned distribution activities. For example, it
highlights enhancements for vaccine distribution that include efforts to
vaccinate high-risk and underserved populations (including ethnic
minority populations and rural communities). The strategy states that, to
date, CDC has provided $3 billion of the at least $4.29 billion it intends to
use as grants to state, local, tribal, and territorial jurisdictions for COVID-
19 vaccine distribution. According to the distribution strategy, details of
CDC’s efforts are still under development and will be included in
subsequent reports to Congress.
We provided a draft of this report to DOD, HHS, and FEMA for review and
comment. DOD, HHS, and FEMA provided technical comments, which
we incorporated as appropriate.
We are sending copies of this report to the appropriate congressional
committees, the Secretary of Health and Human Services, the Secretary
of Defense, FEMA, and other interested parties. In addition, the report is
available at no charge on the GAO website at http://www.gao.gov.
If you or your staff have any questions about this report, please contact
Alyssa M. Hundrup at (202) 512-7114 or [email protected]. Contact
points for our Offices of Congressional Relations and Public Affairs may
be found on the last page of this report. GAO staff who made major
contributions to this report are listed in appendix I.
Alyssa M. Hundrup
Acting Director, Health Care
66
Pub. L. No. 116-260, div. M, tit. III, 134 Stat. 1182, 1912 (2020). This strategy is to be
updated and provided to Congress every 90 days through the end of fiscal year 2021.
Agency Comments
Page 45 GAO-21-443 COVID-19 Vaccines
List of Addressees
The Honorable Patrick Leahy
Chair
The Honorable Richard Shelby
Vice Chairman
Committee on Appropriations
United States Senate
The Honorable Ron Wyden
Chair
The Honorable Mike Crapo
Ranking Member
Committee on Finance
United States Senate
The Honorable Patty Murray
Chair
The Honorable Richard Burr
Ranking Member
Committee on Health, Education, Labor, and Pensions
United States Senate
The Honorable Gary C. Peters
Chair
The Honorable Rob Portman
Ranking Member
Committee on Homeland Security and Governmental Affairs
United States Senate
The Honorable Rosa L. DeLauro
Chairwoman
The Honorable Kay Granger
Ranking Member
Committee on Appropriations
House of Representatives
The Honorable Frank Pallone, Jr.
Chair
The Honorable Cathy McMorris Rodgers
Republican Leader
Committee on Energy and Commerce
House of Representatives

Page 46 GAO-21-443 COVID-19 Vaccines
The Honorable Bennie G. Thompson
Chair
The Honorable John Katko
Ranking Member
Committee on Homeland Security
House of Representatives
The Honorable Carolyn B. Maloney
Chairwoman
The Honorable James Comer
Ranking Member
Committee on Oversight and Reform
House of Representatives
The Honorable Richard E. Neal
Chairman
The Honorable Kevin Brady
Republican Leader
Committee on Ways and Means
House of Representatives
The Honorable James E. Clyburn
Chair
Select Subcommittee on the Coronavirus Crisis
Committee on Oversight and Reform
House of Representatives
The Honorable Bill Foster
House of Representatives
The Honorable Mark E. Green, MD
House of Representatives

Appendix I: GAO Contact and Staff
Acknowledgments
Page 47 GAO-21-443 COVID-19 Vaccines
In addition to the contact named above Kelly DeMots (Assistant Director),
Alison Goetsch (Analyst in Charge), Nora Adkins, Darnita Akers, Jennie
Apter, La Sherri Bush, Kaitlin Farquharson, Ryan Han, Nicolaus Heun,
Katheryn Hubbell, Gay Hee Lee, Jason Lee, Jeff Mayhew, Linda McIver,
Christopher Murray, Patrick Netherclift, Angie Nichols-Friedman, Miranda
Riemer, Ethiene Salgado-Rodriquez, Fatima Sharif, Meghan Shrewsbury,
Will Simerl, and Kim Yamane made key contributions to this report.
Appendix I: GAO Contact and Staff
Acknowledgments
GAO Contact
Staff
Acknowledgments

Related GAO Products
Page 48 GAO-21-443 COVID-19 Vaccines
COVID-19: Sustained Federal Action is Crucial as Pandemic Enters Its
Second Year. GAO-21-387. Washington, D.C.: March 31, 2021.
Operation Warp Speed: Accelerated COVID-19 Vaccine Development
Status and Manufacturing Challenges. GAO-21-319. Washington, D.C.:
February 11, 2021.
Operation Warp Speed Dashboard, https://ows.gaoinnovations.gov/
COVID-19: Critical Vaccine Distribution, Supply Chain, Program Integrity,
and Other Challenges Require Focused Federal Attention. GAO-21-265.
Washington, D.C.: January 28, 2021.
COVID-19: Urgent Actions Needed to Better Ensure an Effective Federal
Response. GAO-21-191. Washington, D.C.: November 30, 2020.
COVID-19: Federal Efforts Accelerate Vaccine and Therapeutic
Development, but More Transparency Needed on Emergency Use
Authorizations. GAO-21-207. Washington, D.C.: November 17, 2020.
COVID-19: Federal Efforts Could Be Strengthened by Timely and
Concerted Actions. GAO-20-701. Washington, D.C.: September 21, 2020.
COVID-19: Opportunities to Improve Federal Response and Recovery
Efforts. GAO-20-625. Washington, D.C.: June 25, 2020.
Related GAO Products
(104841)
The Government Accountability Office, the audit, evaluation, and investigative
arm of Congress, exists to support Congress in meeting its constitutional
responsibilities and to help improve the performance and accountability of the
federal government for the American people. GAO examines the use of public
funds; evaluates federal programs and policies; and provides analyses,
recommendations, and other assistance to help Congress make informed
oversight, policy, and funding decisions. GAO’s commitment to good government
is reflected in its core values of accountability, integrity, and reliability.
The fastest and easiest way to obtain copies of GAO documents at no cost is
through our website. Each weekday afternoon, GAO posts on its website newly
released reports, testimony, and correspondence. You can also subscribe to
GAO’s email updates to receive notification of newly posted products.
The price of each GAO publication reflects GAO’s actual cost of production and
distribution and depends on the number of pages in the publication and whether
the publication is printed in color or black and white. Pricing and ordering
information is posted on GAO’s website, https://www.gao.gov/ordering.htm.
Place orders by calling (202) 512-6000, toll free (866) 801-7077, or
TDD (202) 512-2537.
Orders may be paid for using American Express, Discover Card, MasterCard,
Visa, check, or money order. Call for additional information.
Connect with GAO on Facebook, Flickr, Twitter, and YouTube.
Subscribe to our RSS Feeds or Email Updates. Listen to our Podcasts.
Visit GAO on the web at https://www.gao.gov.
Contact FraudNet:
Website: https://www.gao.gov/fraudnet/fraudnet.htm
Automated answering system: (800) 424-5454 or (202) 512-7700
Orice Williams Brown, Managing Director, [email protected]ov, (202) 512-4400,
U.S. Government Accountability Office, 441 G Street NW, Room 7125,
Washington, DC 20548
Chuck Young, Managing Director, young[email protected], (202) 512-4800
U.S. Government Accountability Office, 441 G Street NW, Room 7149
Washington, DC 20548
Stephen J. Sanford, Acting Managing Director, spel@gao.gov, (202) 512-4707
U.S. Government Accountability Office, 441 G Street NW, Room 7814,
Washington, DC 20548
GAO’s Mission
Obtaining Copies of
GAO Reports and
Testimony
Order by Phone
Connect with GAO
To Report Fraud,
Waste, and Abuse in
Federal Programs
Congressional
Relations
Public Affairs
Strategic Planning and
External Liaison
Please Print on Recycled Paper.
